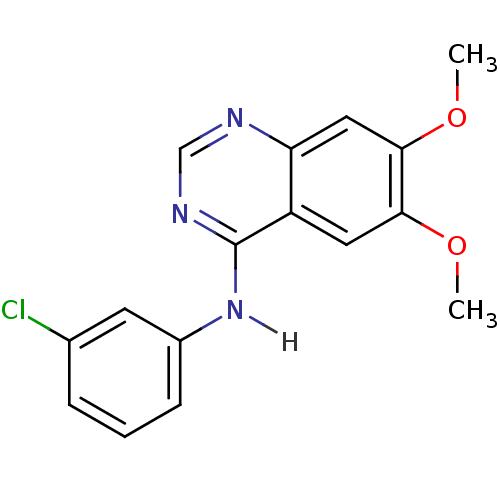
BDBM3532 CHEMBL540068::CHEMBL7917::N-(3-chlorophenyl)-6,7-dimethoxyquinazolin-4-amine::PD153035 Analog 31
SMILES COc1cc2c(cc1OC)ncnc2Nc3cccc(c3)Cl
InChI Key InChIKey=GFNNBHLJANVSQV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 3532
Found 32 hits for monomerid = 3532
Affinity DataIC50: 0.310nMAssay Description:IC50 is the inhibitor concentration which inhibits 50% of kinase activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] labe...More data for this Ligand-Target Pair
Affinity DataIC50: 0.800nMAssay Description:Inhibition of human EGFR tyrosine kinase phosphorylation expressed in mouse BaF3 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.26nMAssay Description:Displacement of [125I]4-(3-iodoanilino)-6,7-dimethoxyquinazoline from EGFR tyrosine kinase in human A431 cell membranesMore data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibitory concentration against Epidermal growth factor receptor tyrosine kinase activityMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of Epidermal growth factor receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of Epidermal growth factor receptor autophosphorylation, 0.05-0.10More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Inhibition of EGF-dependent mouse keratinocyte MK cell proliferationMore data for this Ligand-Target Pair
Affinity DataIC50: 120nMAssay Description:Inhibition of Epidermal growth factor receptor-dependent phosphorylationMore data for this Ligand-Target Pair
Affinity DataIC50: 125nMAssay Description:Inhibition of v-Abl tyrosine kinaseMore data for this Ligand-Target Pair
TargetMAP kinase-interacting serine/threonine-protein kinase 1(Human)
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Spanish National Cancer Research Centre (Cnio)
Curated by ChEMBL
Affinity DataIC50: 560nMAssay Description:Inhibitory concentration against alpha mitogen activated protein kinase p38 activityMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMpH: 7.6 T: 2°CAssay Description:The activity of EGFR, preactivated with EGF, is measured by its ability to transfer terminal phosphate from [gamma-32P]ATP to poly(GAT) substrate.More data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+3nMAssay Description:Concentration required to inhibit the human liver recombinant fructose-1,6-bisphosphatase.More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.87E+3nMAssay Description:Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 1.87E+3nMAssay Description:Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+3nMAssay Description:Inhibition of p60 c-Src tyrosine kinaseMore data for this Ligand-Target Pair
TargetAlpha-ketoglutarate-dependent dioxygenase FTO(Human)
University College London
Curated by ChEMBL
University College London
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of FTO (unknown origin) demethylation activity using m6A7-Broccoli RNA as substrate incubated for 3 mins under shaking condition and measu...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+3nMAssay Description:Inhibition of p56lck kinase autophosphorylation in Jurkat cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Compound was evaluated for its concentration required to inhibit the rat liver F16BPaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Compound was evaluated for its concentration required to inhibit the porcine kidney F16BPaseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of Platelet-derived growth factor receptor-dependent phosphorylationMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 1.96E+4nMAssay Description:Displacement of (6-FAM)KI(pY)VV from full length PKMYT1 (unknown origin) by fluorescence polarization immuno assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 1.96E+4nMAssay Description:Inhibition of human full length PKMYT1 expressed in HEK293 cells using EFS (247 to 259 residues) as substrate after 1 hr by fluorescence polarization...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.62E+4nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.62E+4nMAssay Description:Inhibition of N-[3',6'-dihydroxy-3-oxo-3H-spiro(2-benzofuran-1,9'-xanthen)-5-yl]-N'-[2-(4-{4-[N-(2-chloro-6-methylphenyl)-5-carboxamido]-thiazol-2-yl...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataKi: 2.63E+4nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:Displacement of (6-FAM)KI(pY)VV from PKMYT1 kinase domain (unknown origin) by fluorescence polarization binding assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Martin-Luther-University Halle-Wittenberg
Curated by ChEMBL
Affinity DataIC50: 5.50E+4nMAssay Description:Inhibition of N-[3',6'-dihydroxy-3-oxo-3H-spiro(2-benzofuran-1,9'-xanthen)-5-yl]-N'-[2-(4-{4-[N-(2-chloro-6-methylphenyl)-5-carboxamido]-thiazol-2-yl...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of Protein kinase C alphaMore data for this Ligand-Target Pair