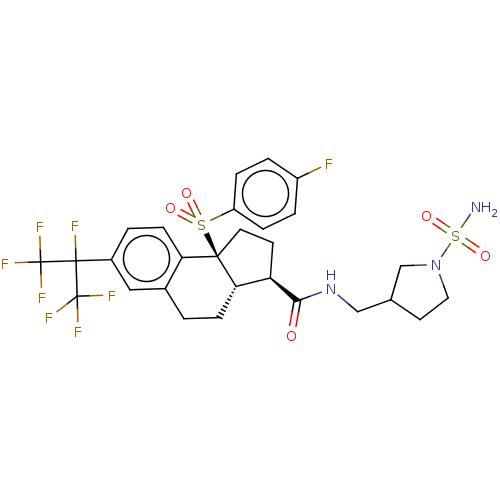
BDBM414395 US10435369, Example 282::US10435369, Example 283
SMILES NS(=O)(=O)N1CCC(CNC(=O)[C@@H]2CC[C@@]3([C@H]2CCc2cc(ccc32)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c2ccc(F)cc2)C1
InChI Key InChIKey=RZPHHKFIKRNNEB-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 414395
Found 2 hits for monomerid = 414395
Affinity DataIC50: 6nMAssay Description:Inverse agonist activity of potential ligands to RORγ was measured by inhibition of luminescence in a Gal4-luciferase reporter assay in Jurkat c...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity of potential ligands to RORγ was measured by inhibition of luminescence in a Gal4-luciferase reporter assay in Jurkat c...More data for this Ligand-Target Pair