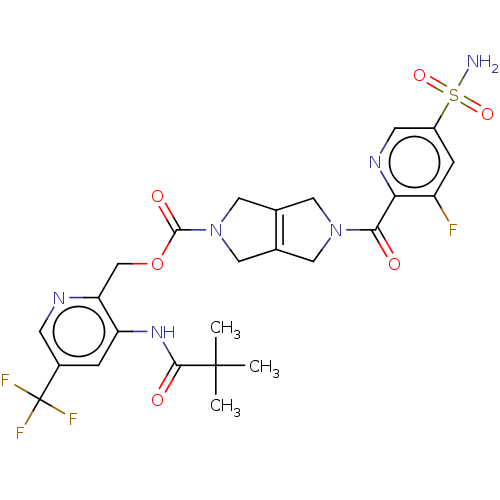
BDBM442736 4-[5-[2-cyclopropyl-6-(oxan-4-::US10647719, Example 1.33
SMILES CC(C)(C)C(=O)Nc1cc(cnc1COC(=O)N1CC2=C(C1)CN(C2)C(=O)c1ncc(cc1F)S(N)(=O)=O)C(F)(F)F
InChI Key InChIKey=GQDFANKSIDIEMO-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 442736
Found 2 hits for monomerid = 442736
Affinity DataIC50: 1nMAssay Description:ATX inhibition was measured by a fluorescence quenching assay using a specifically labeled substrate analogue (MR121 substrate). To obtain this MR121...More data for this Ligand-Target Pair
Affinity DataIC50: 2.30nMAssay Description:Human carbonic anhydrase II (hCA-II) inhibition was measured by an absorbance method using 4-nitrophenyl acetate (4-NPA) as its substrate. 4-NPA can ...More data for this Ligand-Target Pair