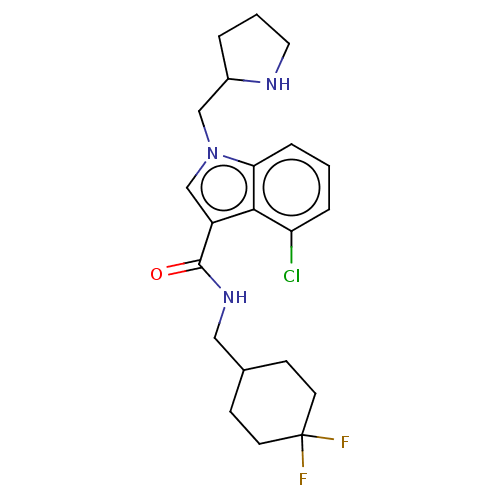
BDBM450448 US10676433, Compound 158::US10676433, Compound 76
SMILES FC1(F)CCC(CNC(=O)c2cn(CC3CCCN3)c3cccc(Cl)c23)CC1
InChI Key InChIKey=LIWOJUJKVLFKLS-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 450448
Found 3 hits for monomerid = 450448
Affinity DataIC50: 10nMAssay Description:Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor....More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to a...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Agonist-induced pore formation was determined by measuring cellular uptake of YO PRO fluorescence dye in HEK293 transfected with human P2X7 receptor....More data for this Ligand-Target Pair