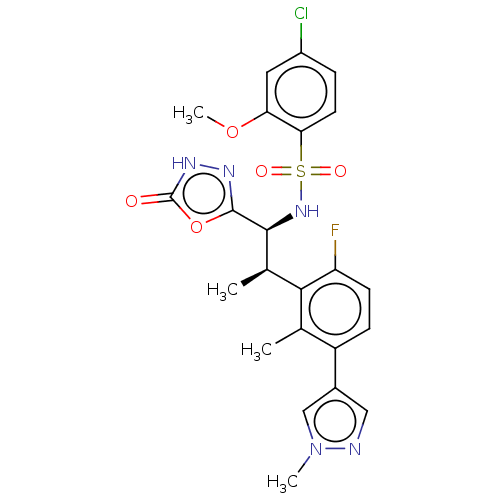
BDBM477533 US10889555, Example 305::US10889555, Example 306::US11634395, Example 306
SMILES COc1cc(Cl)ccc1S(=O)(=O)N[C@@H]([C@H](C)c1c(F)ccc(-c2cnn(C)c2)c1C)c1n[nH]c(=O)o1
InChI Key InChIKey=UOLFNMRHECBLBA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 477533
Found 3 hits for monomerid = 477533
Affinity DataIC50: 90nMAssay Description:First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ...More data for this Ligand-Target Pair
TargetRibonucleoside-diphosphate reductase large subunit/subunit M2(Human)
Taiho Pharmaceutial
US Patent
Taiho Pharmaceutial
US Patent
Affinity DataIC50: 90nMAssay Description:The inhibitory activity against the ribonucleotide reduction reaction (hereinafter referred to as RNR reaction) of the test compound was determined b...More data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:First, test compounds were serially diluted with DMSO. Next, human M1 protein and human M2 protein were added to an aqueous albumin solution derived ...More data for this Ligand-Target Pair