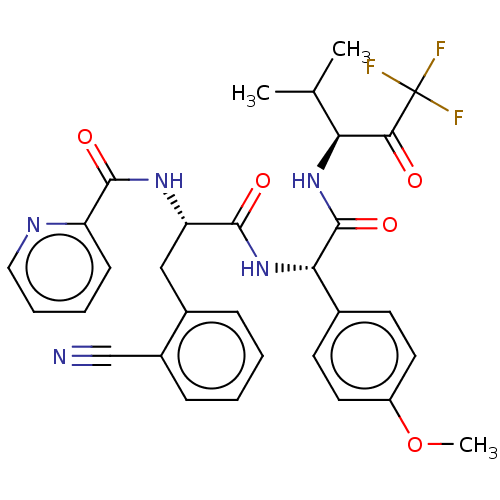
BDBM499102 US11014963, Example 13::US11014963, Example 14
SMILES COc1ccc(cc1)[C@H](NC(=O)[C@H](Cc1ccccc1C#N)NC(=O)c1ccccn1)C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)F
InChI Key InChIKey=GCPVZBQKNVCOHO-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 499102
Found 2 hits for monomerid = 499102
Affinity DataIC50: 1.10nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair
Affinity DataIC50: 4.90nMAssay Description:Enzyme activity is measured by observing the increase in fluorescence intensity caused by cleavage of a peptide substrate containing a fluorophore, w...More data for this Ligand-Target Pair