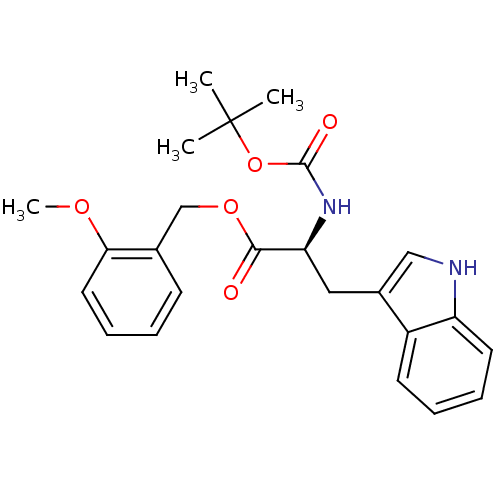
BDBM50030138 (S)-2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-propionic acid 2-methoxy-benzyl ester::2-tert-Butoxycarbonylamino-3-(1H-indol-3-yl)-propionic acid 2-methoxy-benzyl ester::CHEMBL24314
SMILES COc1ccccc1COC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)OC(C)(C)C
InChI Key InChIKey=VCMDLMHMVMHIKF-FQEVSTJZSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50030138
Found 4 hits for monomerid = 50030138
Affinity DataIC50: 280nMAssay Description:Binding affinity towards cloned neurokinin 1 receptor, based on the displacement of [125I]-labeled substance PMore data for this Ligand-Target Pair
Affinity DataIC50: 280nMAssay Description:Tested against cloned human NK1 receptor by displacement of 125 I -labeled substance P expressed in CHO cellsMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Binding affinity to the NK2 receptor assayed by displacement of [125I]-Neurokinin AMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+3nMAssay Description:Affinity to NK3 receptors assayed by displacement of [125I]-Bolton-Hunter labeled eledoisin radioligandMore data for this Ligand-Target Pair