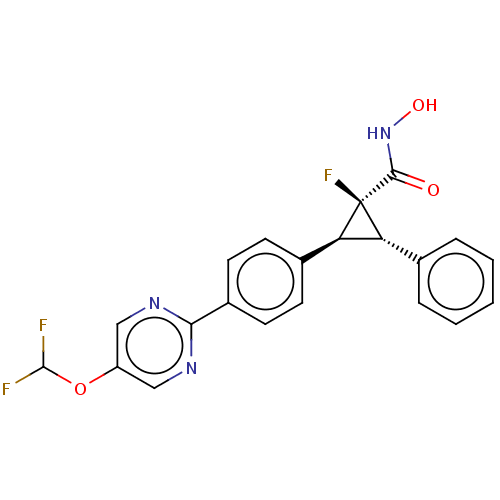
BDBM50160874 CHEMBL3794485::US9505736, (1S,2S,3S)-2-(4-(5- (Difluoromethoxy)pyrimidin-2-yl)phenyl)-1- fluoro-N-hydroxy-3- phenylcyclopropanecarboxamide
SMILES ONC(=O)[C@]1(F)[C@@H]([C@H]1c1ccc(cc1)-c1ncc(OC(F)F)cn1)c1ccccc1
InChI Key InChIKey=RUTGGTYDBCHEPE-LZJOCLMNSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50160874
Found 3 hits for monomerid = 50160874
Affinity DataIC50: 40nMpH: 8.0 T: 25°CAssay Description:The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati...More data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of HDAC4 in human Jurkat E6-1 cells using Lys-TFA as substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of HDAC4 catalytic domain (unknown origin) using Boc-Lys(TFA)-AMC as substrateMore data for this Ligand-Target Pair