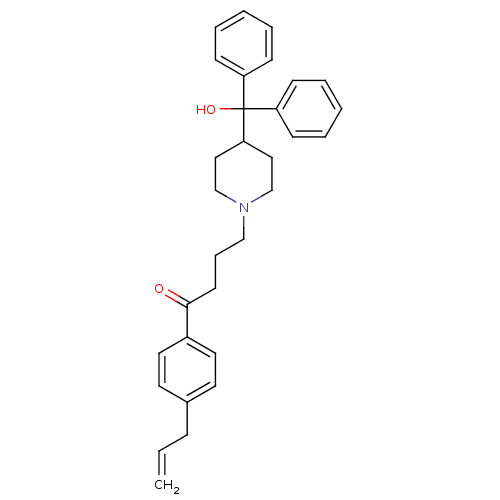
BDBM50183075 1-(4-allylphenyl)-4-(4-(hydroxydiphenylmethyl)piperidin-1-yl)butan-1-one::CHEMBL207831
SMILES OC(C1CCN(CCCC(=O)c2ccc(CC=C)cc2)CC1)(c1ccccc1)c1ccccc1
InChI Key InChIKey=SJXXIFKOMSEXRL-UHFFFAOYSA-N
Data 5 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50183075
Found 5 hits for monomerid = 50183075
Affinity DataIC50: 400nMAssay Description:Inhibition of human recombinant CYP2J2 expressed in baculovirus-infected Sf9 insect cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of human recombinant CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of human recombinant CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of human recombinant CYP2B6More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human recombinant CYP2C8More data for this Ligand-Target Pair