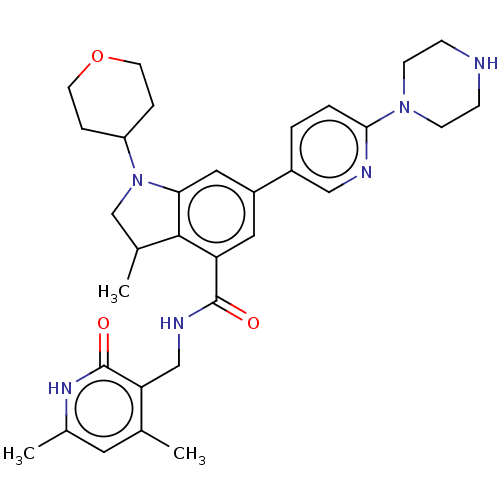
BDBM50205626 CHEMBL3900506
SMILES CC1CN(C2CCOCC2)c2cc(cc(C(=O)NCc3c(C)cc(C)[nH]c3=O)c12)-c1ccc(nc1)N1CCNCC1
InChI Key InChIKey=IGEIYKAMTWPMSB-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50205626
Found 4 hits for monomerid = 50205626
Affinity DataIC50: 2.15E+3nMAssay Description:Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of EZH2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.98E+3nMAssay Description:Inhibition of human EZH1 complex using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.15E+3nMAssay Description:Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysisMore data for this Ligand-Target Pair