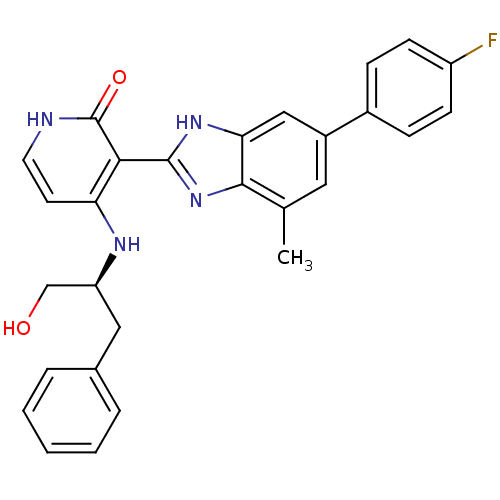
BDBM50209383 (S)-3-(6-(4-fluorophenyl)-4-methyl-1H-benzo[d]imidazol-2-yl)-4-(1-hydroxy-3-phenylpropan-2-ylamino)pyridin-2(1H)-one::CHEMBL230308
SMILES Cc1cc(cc2[nH]c(nc12)-c1c(N[C@H](CO)Cc2ccccc2)cc[nH]c1=O)-c1ccc(F)cc1
InChI Key InChIKey=AKIQDXURYNYZSE-QFIPXVFZSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50209383
Found 4 hits for monomerid = 50209383
TargetInsulin-like growth factor 1 receptor(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 5.10E+3nMAssay Description:Inhibition of human IGF1R expressed in recombinant insect cellsMore data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 6.30E+3nMAssay Description:Inhibition of CYP3A4 in microsomesMore data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 3.20E+3nMAssay Description:Inhibition of CYP1A2 in microsomesMore data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of CYP2C9 in microsomesMore data for this Ligand-Target Pair