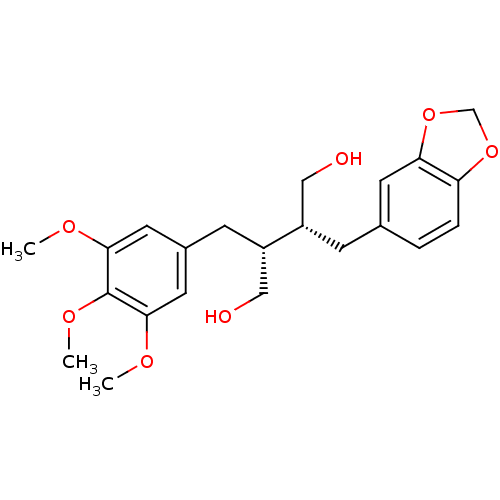
BDBM50259874 (-)-dihydroclusin::CHEMBL469916
SMILES COc1cc(C[C@@H](CO)[C@H](CO)Cc2ccc3OCOc3c2)cc(OC)c1OC
InChI Key InChIKey=FDHFWHRGVDRJIK-IRXDYDNUSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50259874
Found 3 hits for monomerid = 50259874
Affinity DataKi: 54nMAssay Description:Mechanism based inhibition of human cytochrome P450 3A4 measured by (14C)formaldehyde production from (N-methyl-14C)-erythromycinMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human liver microsome CYP2D6 in assessed as [14C]formaldehyde formationMore data for this Ligand-Target Pair
Affinity DataIC50: 800nMAssay Description:Inhibition of human liver microsome CYP3A4 in assessed as [14C]formaldehyde formationMore data for this Ligand-Target Pair