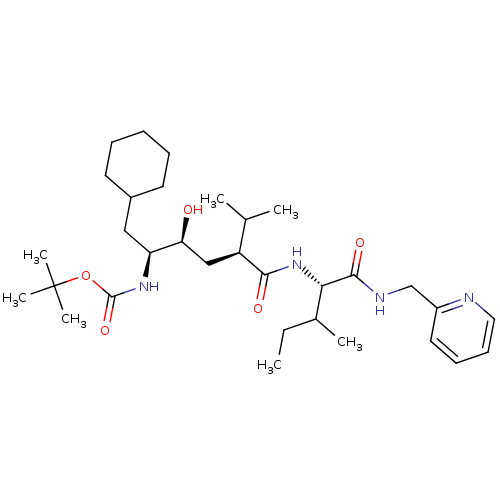
BDBM50281642 ((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-4-{(S)-2-methyl-1-[(pyridin-2-ylmethyl)-carbamoyl]-butylcarbamoyl}-hexyl)-carbamic acid tert-butyl ester::CHEMBL166175
SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1
InChI Key InChIKey=SHXBHHDCBHQKLF-ISYMSECWSA-N
Data 5 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50281642
Found 5 hits for monomerid = 50281642
Affinity DataKi: 8nMAssay Description:Evaluated for inhibitory activity against HIV-1 proteaseMore data for this Ligand-Target Pair
Affinity DataKi: 90nMAssay Description:Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin EMore data for this Ligand-Target Pair
Affinity DataKi: 100nMAssay Description:Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin DMore data for this Ligand-Target Pair
Affinity DataKi: 330nMAssay Description:Compound was evaluated for aspartyl protease inhibition selectivity relative to pepsinMore data for this Ligand-Target Pair
Affinity DataKi: 1.20E+3nMAssay Description:Compound was evaluated for aspartyl protease inhibition selectivity relative to reninMore data for this Ligand-Target Pair