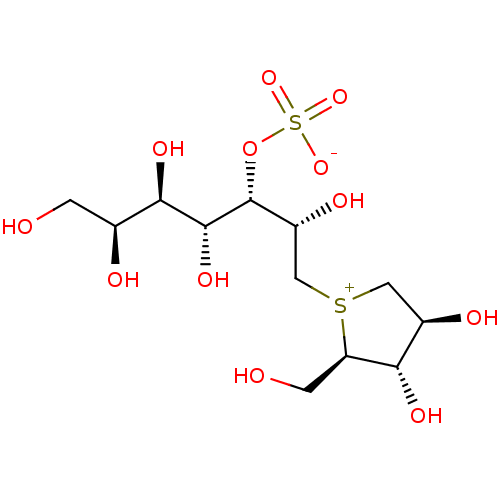
BDBM50316179 (1S,2R,3R,4S)-1-{(1S)-2-[(2R,3S,4S)-3,4-dihydroxy-2-(hydroxymethyl)tetrahydrothiophenium-1-yl]-1-hydroxyethyl}-2,3,4,5-tetrahydroxypentyl sulfate::1,4-Dideoxy-1,4-[[2S,3S,4R,5R,6S-2,4,5,6,7-pentahydroxy-3-(sulfooxy)heptyl]-(R-)epi-sulfoniumylidine]-D-arabinitol::CHEMBL1093264
SMILES OC[C@H](O)[C@@H](O)[C@@H](O)[C@H](OS([O-])(=O)=O)[C@H](O)C[S+]1C[C@@H](O)[C@H](O)[C@H]1CO
InChI Key InChIKey=OMKXVFDVAGCPBS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50316179
Found 17 hits for monomerid = 50316179
Affinity DataKi: 190nMAssay Description:Inhibition of recombinant human maltase glucoamylase N-terminal catalytic domainMore data for this Ligand-Target Pair
Affinity DataKi: 190nMAssay Description:Inhibition of human recombinant N-terminal subunit of maltase-glucoamylase after 60 mins by glucose oxidase assayMore data for this Ligand-Target Pair
Affinity DataKi: 420nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 540nMAssay Description:Inhibition of rat intestinal maltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of rat small intestinal sucrase after 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of rat small intestinal sucrase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 750nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.37E+3nMAssay Description:Inhibition of rat intestinal sucraseMore data for this Ligand-Target Pair
Affinity DataKi: 4.20E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.48E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of rat intestinal isomaltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of rat small intestinal isomaltase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.70E+3nMAssay Description:Inhibition of rat small intestinal isomaltase after 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.60E+3nMAssay Description:Inhibition of rat intestinal maltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of rat small intestinal maltase after 30 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of rat intestinal maltaseMore data for this Ligand-Target Pair
Affinity DataIC50: 7.20E+3nMAssay Description:Inhibition of rat small intestinal maltase after 30 mins by glucose-oxidase methodMore data for this Ligand-Target Pair