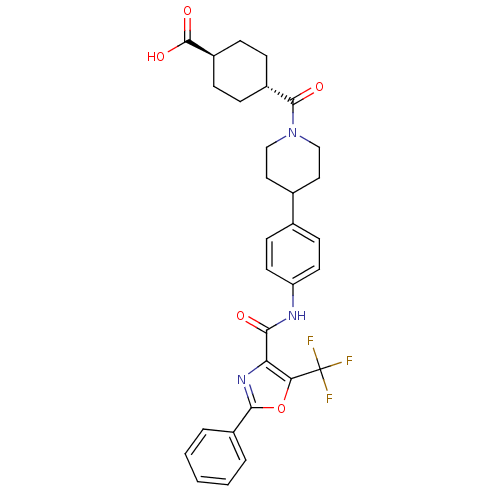
BDBM50341782 4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carbonyl)-amino]phenyl}piperidine-1-carbonyl)-trans-cyclohexanecarboxylic Acid::CHEMBL1766811
SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CCC(CC1)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cc1
InChI Key InChIKey=MARFUFVBECBSBC-HZCBDIJESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50341782
Found 10 hits for monomerid = 50341782
Affinity DataIC50: 114nMAssay Description:Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat...More data for this Ligand-Target Pair
Affinity DataEC50: 493nMAssay Description:Inhibition of human DGAT1 expressed in CHO-K1 cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibition of human DGAT1 expressed in baculovirus infected insect cells after 1 hr using palmitoyl-1-14C-coenzyme A by phospholipid flashplate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 6.84E+4nMAssay Description:Inhibition of human ACAT2 expressed in human HepG2 cells after incubated with compound for 15 mins measured after 1 hr using palmitoyl-1-14C-coenzyme...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of human DGAT2 expressed in baculovirus infected Sf9 cells using palmitoyl-1-14C-coenzyme A by TLC assayMore data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
Affinity DataIC50: 6.84E+4nMAssay Description:Inhibition of human ACAT1 expressed in human HepG2 cells after incubated with compound for 15 mins measured after 1 hr using palmitoyl-1-14C-coenzyme...More data for this Ligand-Target Pair
Affinity DataIC50: >5.00E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair