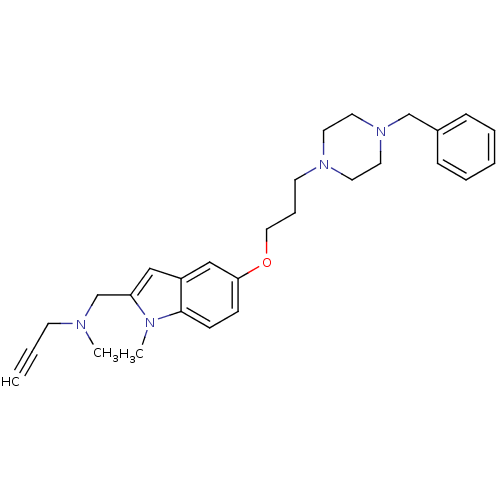
BDBM50359392 CHEMBL1929424::US8999994, 3
SMILES CN(CC#C)Cc1cc2cc(OCCCN3CCN(Cc4ccccc4)CC3)ccc2n1C
InChI Key InChIKey=SLRSNCWUOFPURJ-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50359392
Found 8 hits for monomerid = 50359392
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: >1.00E+5nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetCarboxylic ester hydrolase(Equus caballus (Horse))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 7.60E+3nMpH: 8.0Assay Description:The inhibitory activity of the enzyme acetylcholinesterase (AChE) was evaluated by the Ellman method (Biochem. Pharmacol. 1961, 7, 88) using an elect...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 3.05E+4nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of Electrophorus electricus AchE using acetylthiocholine iodide as substrate preincubated for 10 mins measured after 15 mins of substrate ...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Universitat Aut£Noma De Barcelona
Curated by ChEMBL
Universitat Aut£Noma De Barcelona
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of rat liver mitochondrial MAO-B using [14C]-phenylethylamine after 30 mins by scintillation countingMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Rattus norvegicus (rat))
Consejo Superior De Investigaciones Cientificas
US Patent
Consejo Superior De Investigaciones Cientificas
US Patent
Affinity DataIC50: 31nMAssay Description:Inhibition of rat liver mitochondrial MAO-A using [14C]-5-hydroxytryptamine after 30 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 7.60E+3nMAssay Description:Inhibition of equine serum BuchE using butyrylthiocholine iodide as substrate preincubated for 10 mins measured after 15 mins of substrate addition b...More data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] B(Rattus norvegicus (rat))
Universitat Aut£Noma De Barcelona
Curated by ChEMBL
Universitat Aut£Noma De Barcelona
Curated by ChEMBL
Affinity DataIC50: 1.64E+6nMpH: 7.4 T: 2°CAssay Description:The inhibitory activity of monoamine oxidases A and B was assessed by the Fowler and Tipton radiometric method (Biochem Pharmacol 1981, 30, 3329) usi...More data for this Ligand-Target Pair