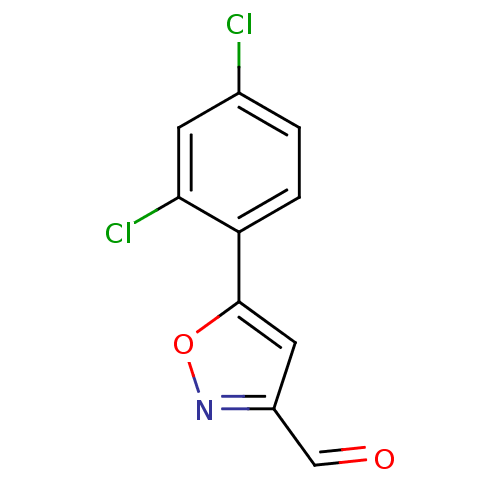
BDBM50382109 CHEMBL2023319
SMILES Clc1ccc(-c2cc(C=O)no2)c(Cl)c1
InChI Key InChIKey=BVUVSNNTQGPIAE-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50382109
Found 4 hits for monomerid = 50382109
Affinity DataIC50: 3.90E+5nMAssay Description:Inhibition of human acrosin using N-alpha-benzoyl-L-arginine p-nitroanilide as substrate after 3 hrs by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.70E+6nMAssay Description:Inhibition of acrosin in human spermatozoa assessed as effect on amidase activity after 3 hrs incubation by spectrophotometryMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+5nMAssay Description:Inhibition of human sperm acrosin using BAPNA as substrate after 3 hrs by spectrophotometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+7nMAssay Description:Inhibition of human acrosin using N-alpha-benzoyl-DL-arginine para-nitroanilide-HCl as substrate after 3 hrs by spectrophotometryMore data for this Ligand-Target Pair