BDBM50382548 CHEMBL2022995
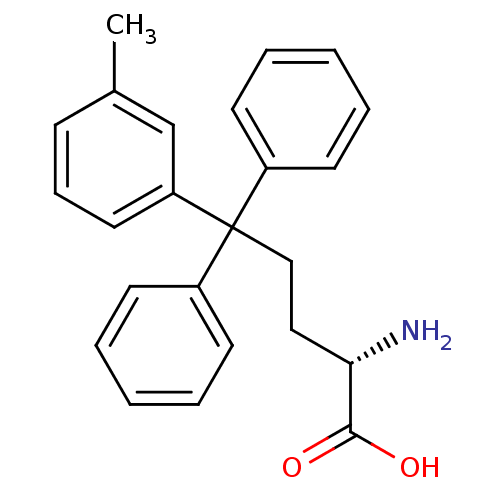
SMILES Cc1cccc(c1)C(CC[C@H](N)C(O)=O)(c1ccccc1)c1ccccc1
InChI Key InChIKey=JXIGVUPYYZGRRZ-QFIPXVFZSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50382548
Found 8 hits for monomerid = 50382548
Affinity DataKi: 11.1nMAssay Description:Inhibition of N-terminal hexa-histidine tagged human cloned Eg5 (1 to 368 amino acids) expressed in Escherichia coli BL21 (DE3) assessed as reduction...More data for this Ligand-Target Pair
Affinity DataKi: 12.8nMAssay Description:Inhibition of N-terminal hexa-histidine tagged human cloned Eg5 (1 to 368 amino acids) expressed in Escherichia coli BL21 (DE3) assessed as reduction...More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2C19More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2D6More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP3A4More data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP1A2More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
The Beatson Institute For Cancer Research
Curated by ChEMBL
The Beatson Institute For Cancer Research
Curated by ChEMBL
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of human ERGMore data for this Ligand-Target Pair
Affinity DataIC50: >2.50E+4nMAssay Description:Inhibition of CYP2C9More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)