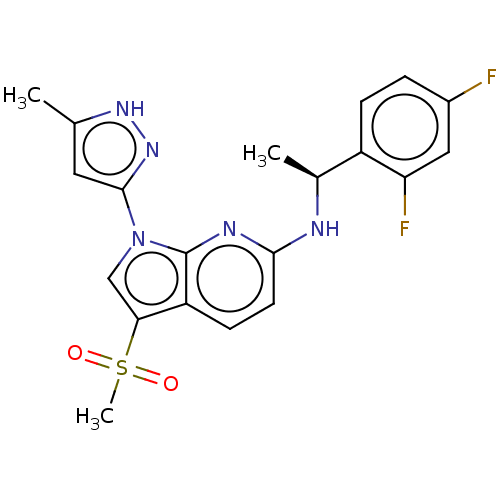
BDBM50524985 CHEMBL4460367
SMILES C[C@H](Nc1ccc2c(cn(-c3cc(C)[nH]n3)c2n1)S(C)(=O)=O)c1ccc(F)cc1F
InChI Key InChIKey=UGWBYFCYZUIYGV-LBPRGKRZSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50524985
Found 3 hits for monomerid = 50524985
Affinity DataIC50: 15nMAssay Description:Inhibition of N-terminal GST-tagged human TrkA kinase domain (436 to 790 residues) expressed in baculovirus expression system using biotin-poly-GT as...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20nMAssay Description:Inhibition of human ALK kinase domain (1058 to 1620 residues) expressed in baculovirus expression system using biotin-poly-GT as substrate pre-incuba...More data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of recombinant human ALK expressed in HEK293 cells assessed as reduction in ALK autophosphorylation at Tyr1604 residue incubated for 60 mi...More data for this Ligand-Target Pair