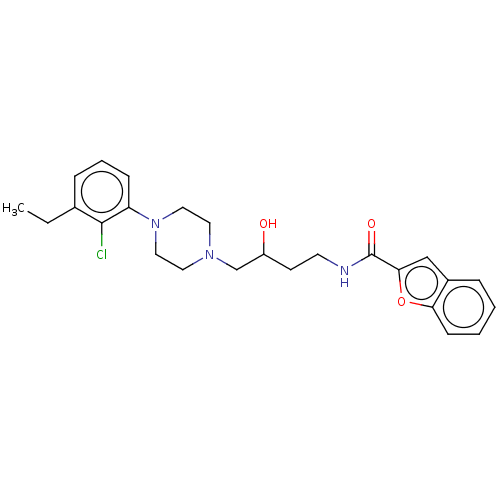
BDBM50533619 CHEMBL4517102
SMILES CCc1cccc(N2CCN(CC(O)CCNC(=O)c3cc4ccccc4o3)CC2)c1Cl
InChI Key InChIKey=ANDAVALYLUYQOC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50533619
Found 9 hits for monomerid = 50533619
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 0.985nMAssay Description:Displacement of [3H]N-methylspiperone from human dopamine D3 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation c...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2A(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 3.20nMAssay Description:Displacement of [125I]DOI from 5-HT2A receptor (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 28nMAssay Description:Displacement of [3H]-8-OH-DPAT from 5-HT1A receptor (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 2C(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 89nMAssay Description:Displacement of [125I]DOI from 5-HT2C receptor (unknown origin)More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataEC50: 118nMAssay Description:Agonist activity at 5-HT1A receptor (unknown origin) by [35S]GTPgammaS binding assayMore data for this Ligand-Target Pair
TargetD(2) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 164nMAssay Description:Displacement of [3H]N-methylspiperone from human dopamine D2L receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation ...More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataEC50: 196nMAssay Description:Agonist activity at human dopamine D3 receptor expressed in CHO cells assessed as stimulation of mitogenesis incubated for 16 hrs in presence of [3H]...More data for this Ligand-Target Pair
TargetD(3) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataIC50: 230nMAssay Description:Antagonist activity at human dopamine D3 receptor expressed in CHO cells assessed as inhibition of quinpirole-stimulated mitogenesis incubated for 16...More data for this Ligand-Target Pair
TargetD(4) dopamine receptor(Human)
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
National Institute On Drug Abuse-Intramural Research Program
Curated by ChEMBL
Affinity DataKi: 2.38E+3nMAssay Description:Displacement of [3H]N-methylspiperone from human dopamine D4.4 receptor expressed in HEK293 cell membranes incubated for 1 hr by liquid scintillation...More data for this Ligand-Target Pair