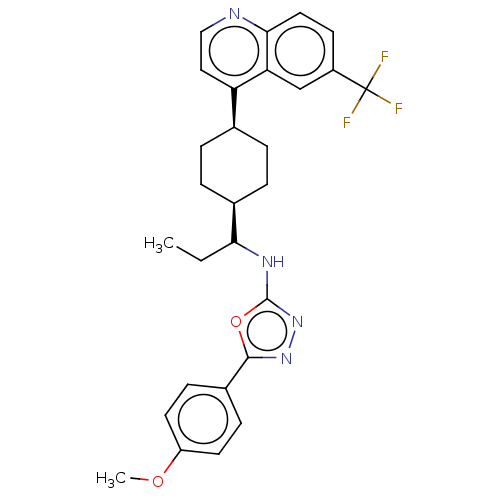
BDBM50559678 CHEMBL4763467
SMILES CCC(Nc1nnc(o1)-c1ccc(OC)cc1)[C@H]1CC[C@H](CC1)c1ccnc2ccc(cc12)C(F)(F)F
InChI Key InChIKey=SUDVIJKGHSMHQM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50559678
Found 4 hits for monomerid = 50559678
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 3nMAssay Description:Inhibition of IDO1 in recombinant IFN-gamma induced human HeLa cells incubated for 18 hrs by fluorescence microplate reader assayMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Mouse)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of IDO1 in recombinant IFN-gamma induced mouse M109 cells incubated for 18 hrs by fluorescence microplate reader assayMore data for this Ligand-Target Pair
TargetIndoleamine 2,3-dioxygenase 1(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataIC50: 50nMAssay Description:Inhibition of IDO1 in IFN-gamma/LPS induced human whole blood assessed as tryptophan/kynurenine level measured after 18 hrs by RapidFire mass spectro...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Bristol Myers Squibb Research and Development
Curated by ChEMBL
Affinity DataEC50: 1.06E+3nMAssay Description:Transactivation of PXR (unknown origin) assessed as CYP450 inductionMore data for this Ligand-Target Pair