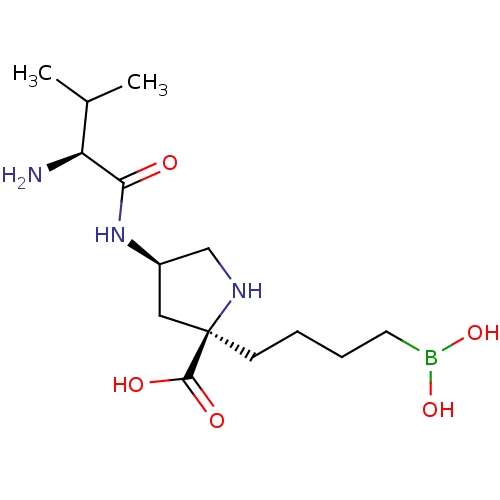
BDBM50561043 CHEMBL4746323::US11420984, Example 9
SMILES CC(C)[C@H](N)C(=O)N[C@H]1CN[C@](CCCCB(O)O)(C1)C(O)=O
InChI Key InChIKey=GRDCIXKDANGFSB-UHIISALHSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50561043
Found 4 hits for monomerid = 50561043
Affinity DataIC50: 320nMAssay Description:Inhibition of human Arg1 by TOGA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ...More data for this Ligand-Target Pair
Affinity DataIC50: 320nMAssay Description:The inhibitory effects of Examples 1 to 30 on the activity of Human Arginase 1 and Arginase 2 activity were quantified by measuring the formation of ...More data for this Ligand-Target Pair
Affinity DataIC50: 330nMAssay Description:Inhibition of human Arg2 by TOGA assayMore data for this Ligand-Target Pair