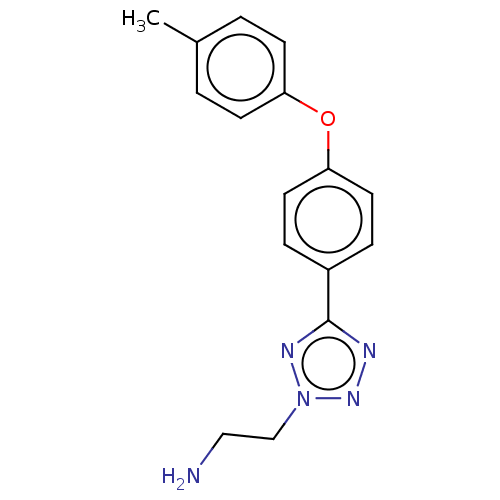
BDBM50575465 CHEMBL4851581
SMILES Cc1ccc(Oc2ccc(cc2)-c2nnn(CCN)n2)cc1
InChI Key InChIKey=DRMJFYFIKJYJRF-UHFFFAOYSA-N
Data 8 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50575465
Found 8 hits for monomerid = 50575465
Affinity DataIC50: 3.60nMAssay Description:Inhibition of LTA4H (unknown origin) using Arg-AMC as substrate preincubated with compound for 15 mins followed by substrate addition and measured af...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of recombinant full length N-terminal His6-tagged LTA4H (unknown origin) expressed in Escherichia coli BL21 DE3 cells at enzyme concentrat...More data for this Ligand-Target Pair
Affinity DataIC50: 121nMAssay Description:Inhibition of LTA4H in human whole blood assessed as reduction of calcium ionophore A23187-stimulated LTB4 generation preincubated for 15 mins follow...More data for this Ligand-Target Pair
Affinity DataIC50: 121nMAssay Description:Inhibition of LTA4H in human whole blood assessed as ionophore-stimulated LTB4 release preincubated for 15 mins by competitive immunoassayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+3nMAssay Description:Reversible inhibition of CYP2C9 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 5.80E+3nMAssay Description:Displacement of [3H]dofetilide from recombinant human ERG stably expressed in HEK293 cell membranes measured after 90 mins by microbeta liquid scinti...More data for this Ligand-Target Pair
Affinity DataIC50: 1.40E+4nMAssay Description:Reversible inhibition of CYP2D6 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH by LC-MS analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Reversible inhibition of CYP3A5 in human liver microsomes using midazolam as substrate incubated for 10 mins in presence of NADPH by LC-MS analysisMore data for this Ligand-Target Pair