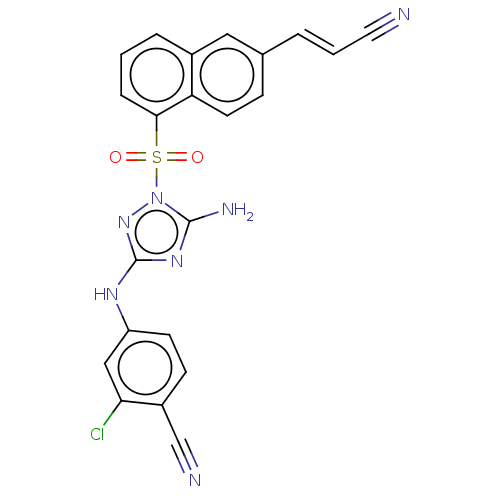
BDBM50621832 CHEMBL5397495
SMILES Nc1nc(Nc2ccc(C#N)c(Cl)c2)nn1S(=O)(=O)c1cccc2cc(\C=C\C#N)ccc12
InChI Key InChIKey=RXXGEBPXQBMTIJ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50621832
Found 3 hits for monomerid = 50621832
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Collaborations Pharmaceuticals
Curated by ChEMBL
Collaborations Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 350nMAssay Description:Inhibition of HIV1 Reverse transcriptase incubated for 30 mins by fluorescence based analysisMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.42E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsomes using diclofenac and sulfaphenazole as substrate preincubated for 5 mins followed by NADPH addition an...More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Collaborations Pharmaceuticals
Curated by ChEMBL
Collaborations Pharmaceuticals
Curated by ChEMBL
Affinity DataIC50: 1.15E+4nMAssay Description:Inhibition of hERG by fluorescence polarization assayMore data for this Ligand-Target Pair
Ligand InfoSimilars