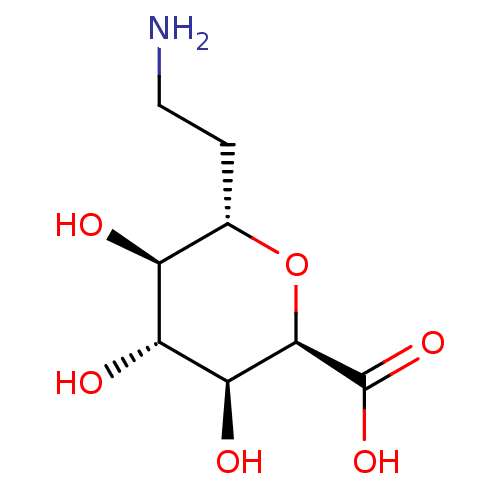
BDBM50625270 CHEMBL5428294::US20250243227, Compound 12
SMILES NCC[C@@H]1O[C@H]([C@@H](O)[C@H](O)[C@H]1O)C(O)=O
InChI Key InChIKey=OFSZGQLGRVXQMI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50625270
Found 3 hits for monomerid = 50625270
Affinity DataKi: 4.00E+3nMAssay Description:Inhibition of human recombinant alpha-L-iduronidase using 4-methylumbelliferyl-alpha-iduronide as fluorogenic substrate assessed as inhibition consta...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataKi: 4.00E+3nM IC50: 6.70E+3nMAssay Description:The compounds of Example 1 were evaluated on their inhibitory potential against rh-α-IDUA, and their apparent IC50 values were determined by using th...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 6.70E+3nMAssay Description:Inhibition of human recombinant alpha-L-iduronidase using 4-methylumbelliferyl-alpha-iduronide as fluorogenic substrateMore data for this Ligand-Target Pair
Ligand InfoSimilars