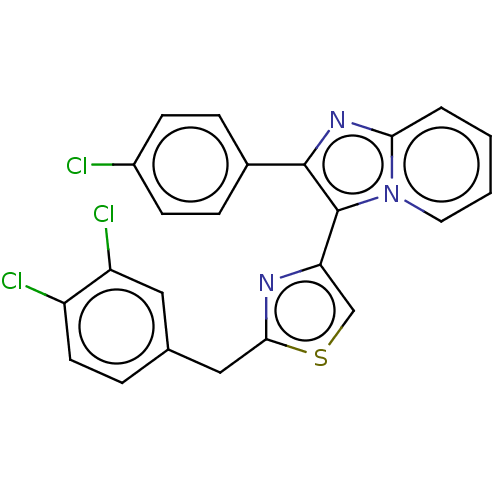
BDBM50632328 CHEMBL5440050
SMILES Clc1ccc(cc1)-c1nc2ccccn2c1-c1csc(Cc2ccc(Cl)c(Cl)c2)n1
InChI Key InChIKey=UDUOPLWXKIPDOP-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50632328
Found 3 hits for monomerid = 50632328
TargetNuclear receptor subfamily 1 group I member 3(Human)
Czech Academy of Sciences
Curated by ChEMBL
Czech Academy of Sciences
Curated by ChEMBL
Affinity DataEC50: 18nMAssay Description:Agonist activity at human recombinant GST-tagged CAR LBD incubated for 1 to 4 hrs in presence of fluorescein-labeled PGC1alpha by lanthascreen TR-FRE...More data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 3(Human)
Czech Academy of Sciences
Curated by ChEMBL
Czech Academy of Sciences
Curated by ChEMBL
Affinity DataEC50: 3.23E+3nMAssay Description:Agonist activity at human CAR LBD in human HepG2 cells incubated for 24 hrs by firefly luciferase reporter assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 1 group I member 2(Human)
Czech Academy of Sciences
Curated by ChEMBL
Czech Academy of Sciences
Curated by ChEMBL
Affinity DataEC50: 2.22E+4nMAssay Description:Activation of PXR in human HepG2 cells incubated for 24 hrs by dual luciferase reporter assayMore data for this Ligand-Target Pair