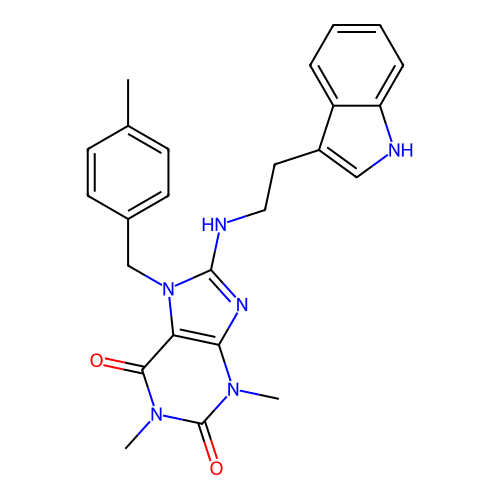
BDBM50635542 CHEMBL5557729
SMILES Cc1ccc(Cn2c(NCCc3c[nH]c4ccccc34)nc3c2c(=O)n(C)c(=O)n3C)cc1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50635542
Found 3 hits for monomerid = 50635542
Affinity DataEC50: 46nMAssay Description:Agonist activity at mouse GPR18 expressed in CHO cells assessed as activation by measuring beta-arrestin2 recruitment incubated for 90 mins by lumine...More data for this Ligand-Target Pair
Affinity DataKi: 827nMAssay Description:Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells assessed as inhibition constant by radioligand competition binding assayMore data for this Ligand-Target Pair
Affinity DataKi: 3.48E+3nMAssay Description:Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells assessed as inhibition constant by radioligand competition binding assayMore data for this Ligand-Target Pair