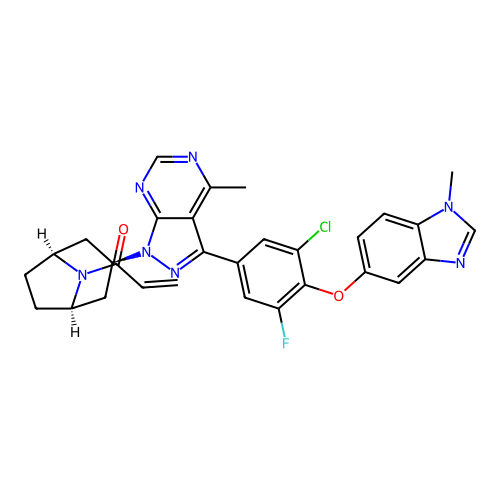
BDBM50637161 CHEMBL5555280
SMILES C=CC(=O)N1[C@@H]2CC[C@H]1C[C@@H](n1nc(-c3cc(F)c(Oc4ccc5c(c4)ncn5C)c(Cl)c3)c3c(C)ncnc31)C2
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50637161
Found 2 hits for monomerid = 50637161
Affinity DataIC50: 49nMAssay Description:Inhibition of wild-type HER2 phosphorylation in human BT-474 cells incubated for 1 hr by In-cell Western assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.58E+3nMAssay Description:Inhibition of wild-type human EGFR phosphorylation expressed in mouse NIH3T3 cells incubated for 1 hr by ELISAMore data for this Ligand-Target Pair