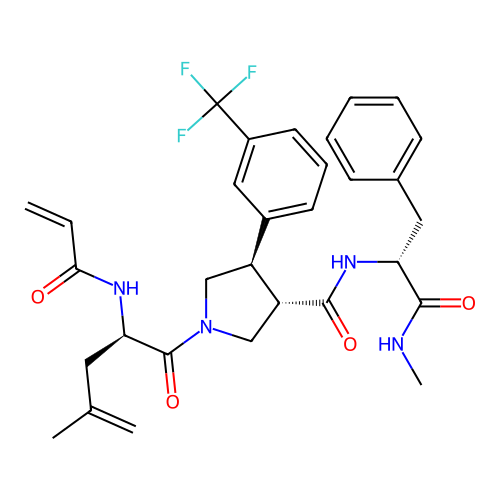
BDBM50642122 CHEMBL5563508
SMILES C=CC(=O)N[C@H](CC(=C)C)C(=O)N1C[C@@H](C(=O)N[C@H](Cc2ccccc2)C(=O)NC)[C@H](c2cccc(C(F)(F)F)c2)C1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50642122
Found 2 hits for monomerid = 50642122
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of SARS-CoV-2 MPro transfected in Escherichia coli BL21 (DE3) measured after 1 hr by FRET analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of SARS-CoV MPro transfected in Escherichia coli BL21 (DE3) using Dabcyl-KTSAVLQSGFRKM-E(Edans)-NH2 as substrate preincubated for 15 mins ...More data for this Ligand-Target Pair