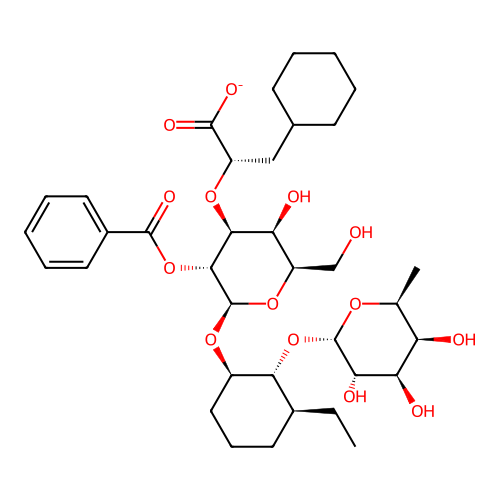
BDBM50646327 CHEMBL5592583
SMILES CC[C@H]1CCC[C@@H](O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O[C@@H](CC3CCCCC3)C(=O)[O-])[C@H]2OC(=O)c2ccccc2)[C@@H]1O[C@@H]1O[C@@H](C)[C@@H](O)[C@@H](O)[C@@H]1O
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50646327
Found 2 hits for monomerid = 50646327
Affinity DataKd: 1.90E+3nMAssay Description:Binding affinity to E-selectin SCR2 domain (unknown origin) by isothermal titration calorimetryMore data for this Ligand-Target Pair
Affinity DataKd: 1.90E+3nMAssay Description:Binding affinity to sialyl LewisX mimetic 21a pretreated Blue-NHS-labelled E-selectin Lec-EGF/SCR1/SCR2 domain (unknown origin) measured after 20 min...More data for this Ligand-Target Pair