BDBM50648020 CHEMBL5597266
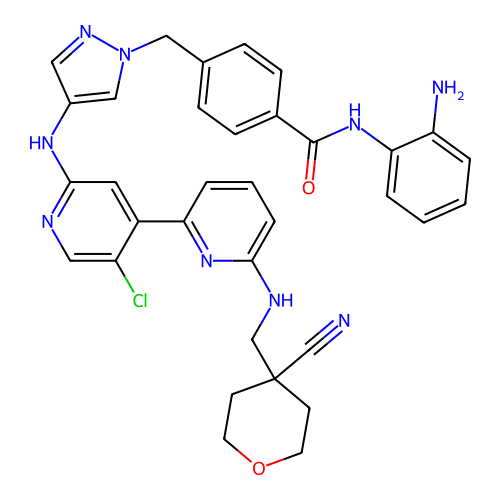
SMILES N#CC1(CNc2cccc(-c3cc(Nc4cnn(Cc5ccc(C(=O)Nc6ccccc6N)cc5)c4)ncc3Cl)n2)CCOCC1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 17 hits for monomerid = 50648020
Found 17 hits for monomerid = 50648020
Affinity DataIC50: 88nMAssay Description:Inhibition of CDK9 (unknown origin) using ULight 4EBP1 peptide as substrate incubated for 2 hrs in presence of ATP by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 169nMAssay Description:Inhibition of human recombinant HDAC1 using Ac-Lys-Tyr-Lys(eta-acetyl)-AMC as substrate incubated for 24 hrs by fluorescence based Envision plate rea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+3nMAssay Description:Inhibition of human recombinant HDAC3 using Ac-Lys-Tyr-Lys(eta-acetyl)-AMC as substrate incubated for 24 hrs by fluorescence based Envision plate rea...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of BTK (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of JAK3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CDK2 (unknown origin) using ULight 4EBP1 peptide as substrate incubated for 2 hrs in presence of ATP by FRET assayMore data for this Ligand-Target Pair
TargetInterleukin-1 receptor-associated kinase 4(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of IRAK4 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase Chk1(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CHK1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of CDK2 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase pim-1(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PIM1 (unknown origin)More data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase 17B(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of DRAK2 (unknown origin)More data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of EGFR (unknown origin)More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein kinase FLT3(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of FLT3 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of AurorA (unknown origin)More data for this Ligand-Target Pair
TargetRibosomal protein S6 kinase beta-1(Human)
University of Chinese Academy of Sciences
Curated by ChEMBL
University of Chinese Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of P70S6K (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human recombinant HDAC4 using Ac-Leu-Gly-Lys(Tfa)-AMC as substrate incubated for 24 hrs by fluorescence based Envision plate reader ana...More data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+4nMAssay Description:Inhibition of human recombinant HDAC6 using Boc-Lys(eta-acetyl)-AMC as substrate incubated for 24 hrs by fluorescence based Envision plate reader ana...More data for this Ligand-Target Pair