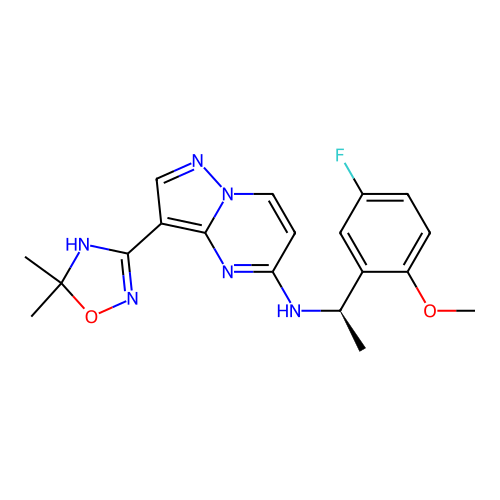
BDBM50649925 CHEMBL5624610::US12435089, Example 10
SMILES COc1ccc(F)cc1[C@@H](C)Nc1ccn2ncc(C3=NOC(C)(C)N3)c2n1
InChI Key
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 12 hits for monomerid = 50649925
Found 12 hits for monomerid = 50649925
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human ROS1 G2032R mutant using KKKSPGEYVNIEFG as substrate incubated for 120 mins in presence of [gamma-33P]-ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Inhibition of human ROS1 using KKKSPGEYVNIEFG as substrate incubated for 120 mins in presence of [gamma-33P]-ATPMore data for this Ligand-Target Pair
Affinity DataIC50: 0.5nMAssay Description:Serial dilutions of the compound were prepared with 10% DMSO, and 5 μl of each dilution was added to a 50 μl reaction vessel to result in a...More data for this Ligand-Target Pair
Affinity DataIC50: 50nMAssay Description:Inhibition of human ALK5 using Casein as substrate incubated for 120 mins in presence of [gamma-33P]-ATPMore data for this Ligand-Target Pair