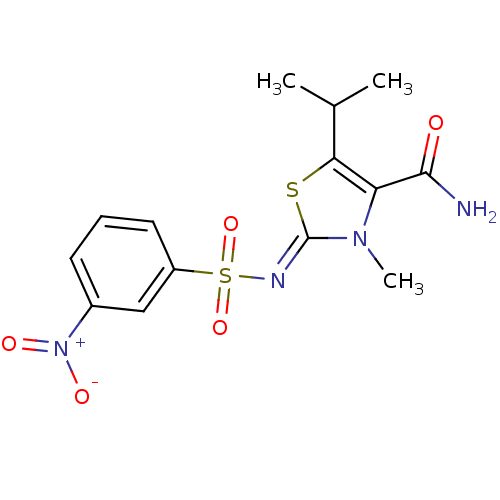
BDBM5121 3-methyl-2-[(3-nitrobenzene)sulfonamido]-5-(propan-2-yl)-2,3-dihydro-1,3-thiazole-4-carboxamide::4- and 5-substituted thiazolidene deriv. 19::5-Isopropyl-3-methyl-2-{[(3-nitrophenyl)sulfonyl]imino}-2,3-dihydro-1,3-thiazole-4-carboxamide
SMILES CC(C)c1s\c(=N/S(=O)(=O)c2cccc(c2)[N+]([O-])=O)n(C)c1C(N)=O
InChI Key InChIKey=GHFIJZDKYNCSQG-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 5121
Found 3 hits for monomerid = 5121
TargetGag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C](Human immunodeficiency virus type 1)
Yamanouchi Pharmaceutical
Yamanouchi Pharmaceutical
Affinity DataIC50: 2.50E+4nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [588-1027]/[588-1147](Human immunodeficiency virus type 1)
Yamanouchi Pharmaceutical
Yamanouchi Pharmaceutical
Affinity DataIC50: 3.90E+4nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N](Human immunodeficiency virus type 1)
Yamanouchi Pharmaceutical
Yamanouchi Pharmaceutical
Affinity DataIC50: 5.00E+4nMAssay Description:The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro.More data for this Ligand-Target Pair