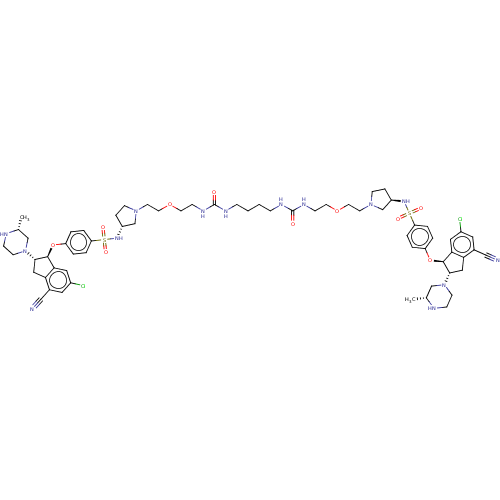
BDBM536030 US11242337, Example 133
SMILES C[C@@H]1CN(CCN1)[C@H]1Cc2c(cc(Cl)cc2C#N)[C@@H]1Oc1ccc(cc1)S(=O)(=O)N[C@@H]1CCN(CCOCCNC(=O)NCCCCNC(=O)NCCOCCN2CC[C@H](C2)NS(=O)(=O)c2ccc(O[C@@H]3[C@H](Cc4c3cc(Cl)cc4C#N)N3CCN[C@H](C)C3)cc2)C1
InChI Key InChIKey=VFLIWYHBFPGPMQ-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 536030
Found 2 hits for monomerid = 536030
Affinity DataIC50: 100nMAssay Description:The ability of compounds to inhibit human and rat NHE-3-mediated Na+ dependent H+ antiport after application and washout was measured using a modific...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:Rat and human NHE-3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensitive dye method originally reported by Paradi...More data for this Ligand-Target Pair