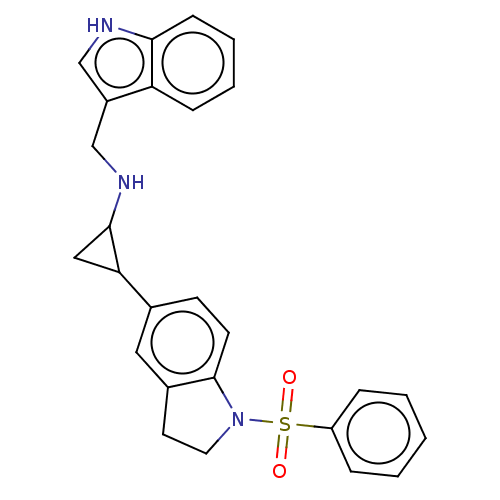
BDBM645356 US11873292, Compound A8::trans-N-(3-indolylmethylene)-2-(1- (phenylsulfonyl)indolin-5-yl) cyclopropylamine
SMILES O=S(=O)(N1CCc2cc(ccc12)C1CC1NCc1c[nH]c2ccccc12)c1ccccc1
InChI Key InChIKey=KNOCVPDEDDCICZ-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 645356
Found 3 hits for monomerid = 645356
Affinity DataIC50: 85.1nMAssay Description:Principle: LSD1 specifically removes the methylation modification at K4 lysine on H3 polypeptide substrate, making it a substrate without methylation...More data for this Ligand-Target Pair
Affinity DataIC50: 8.17E+4nMAssay Description:Principle: A specific luciferin derivative was used as a substrate. MAOA or MAOB can catalyze the conversion of substrate to luciferin methyl ester. ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Principle: A specific luciferin derivative was used as a substrate. MAOA or MAOB can catalyze the conversion of substrate to luciferin methyl ester. ...More data for this Ligand-Target Pair