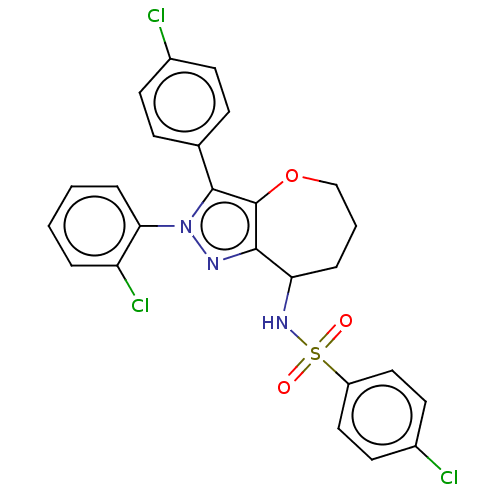
BDBM688323 4-chloro-N-[2-(2-chlorophenyl)-3-(4-chlorophenyl)-5,6,7,8-tetrahydrooxepino[3,2-c]pyrazol-8-yl]benzenesulfonamide::US20240189287, Compound BNS808
SMILES Clc1ccc(cc1)-c1c2OCCCC(NS(=O)(=O)c3ccc(Cl)cc3)c2nn1-c1ccccc1Cl
InChI Key InChIKey=VSSQVUBXWXIWJG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 688323
Found 3 hits for monomerid = 688323
Affinity DataKi: 0.600nMAssay Description:Mouse brain membranes were used as the source material for CB1 receptors. The displacement of specifically bound tritiated CP-55.940 from these membr...More data for this Ligand-Target Pair
Affinity DataKi: 1.93E+3nMAssay Description:Human kidney membranes were used as the source material for CB2 receptors. The displacement of specifically bound tritiated CP-55,940 from these memb...More data for this Ligand-Target Pair
Affinity DataIC50: 5.39E+3nMAssay Description:Cardiotoxicity potential of BNS808 and BNS822 on the hERG potassium channels was evaluated using the automated patch clamp method (SyncroPatch 384PE)...More data for this Ligand-Target Pair