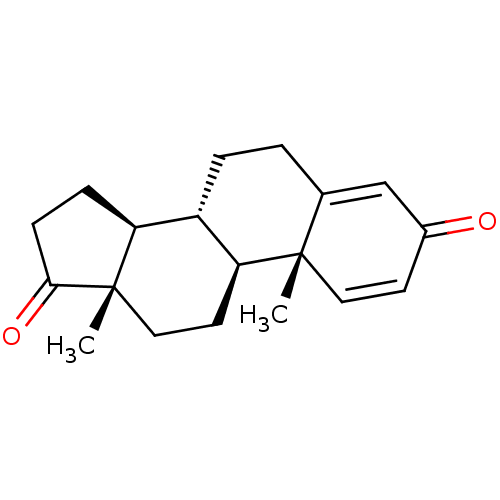
BDBM91718 Androst-1,4-dien-3,17-dione, 7
SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)C=C[C@]34C)[C@@H]1CCC2=O
InChI Key InChIKey=LUJVUUWNAPIQQI-QAGGRKNESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 91718
Found 4 hits for monomerid = 91718
Affinity DataKi: 120nMAssay Description:Inactivation rate (Ki) for human placental aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 260nMAssay Description:Inactivation rate (Ki) for human placental aromatase Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataKi: 320nMAssay Description:Binding affinity was measured on Cytochrome P450 19A1More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+5nMAssay Description:AChE and BChE inhibiting activities were measured in vitro by a modified spectrophotometric method previously developed by Ellman et. al.More data for this Ligand-Target Pair