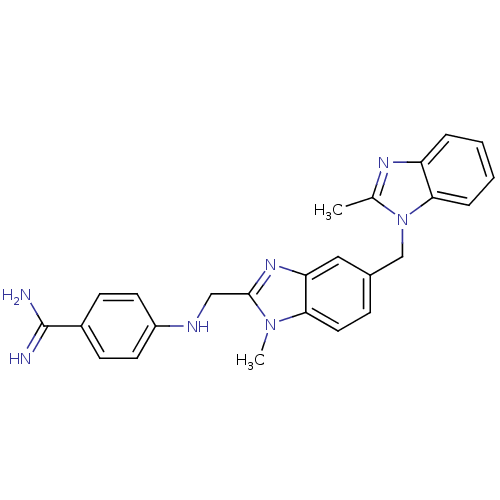
BDBM17297 4-[({1-methyl-5-[(2-methyl-1H-1,3-benzodiazol-1-yl)methyl]-1H-1,3-benzodiazol-2-yl}methyl)amino]benzene-1-carboximidamide::BIBR1109
SMILES Cc1nc2ccccc2n1Cc1ccc2n(C)c(CNc3ccc(cc3)C(N)=N)nc2c1
InChI Key InChIKey=IRKPNOLLMNHSOU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 17297
Found 8 hits for monomerid = 17297
Affinity DataKi: 40nM ΔG°: -10.5kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: 67nM ΔG°: -10.2kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: 780nM ΔG°: -8.66kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: 4.10E+3nM ΔG°: -7.64kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: 9.20E+3nM ΔG°: -7.14kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+4nM ΔG°: -6.80kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: >4.00E+4nM ΔG°: >-6.24kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
Affinity DataKi: >5.00E+4nM ΔG°: >-6.10kcal/molepH: 8.0 T: 2°CAssay Description:For determination of the inhibition constants (Ki), enzyme inhibition was studied at three different substrate concentrations and seven different con...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)