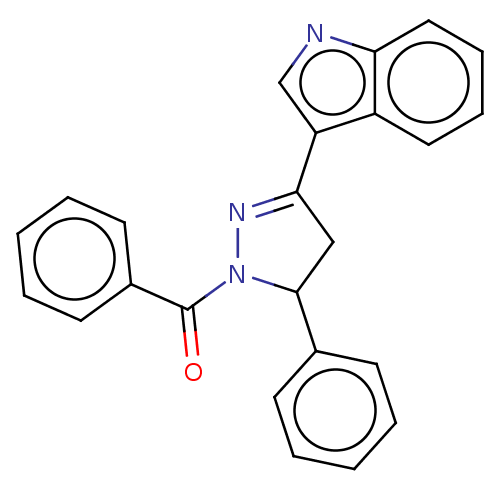
BDBM192116 (3-(1H-indol-3-yl)-5-phenyl-4,5-dihydropyrazol-1-yl)(phenyl) methanone (P3)
SMILES O=C(N1N=C(CC1c1ccccc1)c1cnc2ccccc12)c1ccccc1
InChI Key
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 192116
Found 3 hits for monomerid = 192116
Affinity DataIC50: 1.35E+4nMT: 25°CAssay Description:Preparation of DPPH solution was adopted from Molyneux [J. Sci. Technol. 26(2):211-219] and Blois [Nature 181(4617):1199-1200] with minor modificatio...More data for this Ligand-Target Pair
Affinity DataIC50: 740nMpH: 8.0 T: 25°CAssay Description:The in vitro AChE inhibitory activity was measured using the methods described earlier [Biochem. Pharmacol. 7:88-95]. Briefly, stock solutions (1mg/m...More data for this Ligand-Target Pair
Affinity DataIC50: 1.31E+4nMT: 25°CAssay Description:The ABTS free radical cation scavenging ability of the synthe- sized compounds was determined according to the procedure described earlier [Free Radi...More data for this Ligand-Target Pair