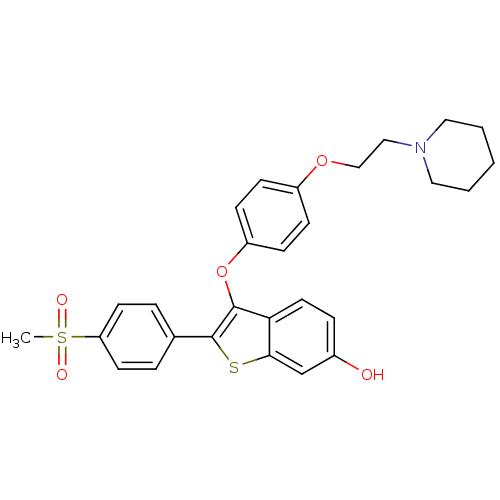
BDBM19447 2-(4-methanesulfonylphenyl)-3-{4-[2-(piperidin-1-yl)ethoxy]phenoxy}-1-benzothiophen-6-ol::Arzoxifene analogue, 13::CHEMBL389907
SMILES CS(=O)(=O)c1ccc(cc1)-c1sc2cc(O)ccc2c1Oc1ccc(OCCN2CCCCC2)cc1
InChI Key InChIKey=KUWSWTJKKAYVBV-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 19447
Found 4 hits for monomerid = 19447
Affinity DataKi: 0.630nMAssay Description:Displacement of [3H]estradiol from human recombinant ERalphaMore data for this Ligand-Target Pair
Affinity DataKi: 13.6nMAssay Description:Displacement of [3H]estradiol from human recombinant ERbetaMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMpH: 7.5 T: 2°CAssay Description:Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMpH: 7.5 T: 2°CAssay Description:Arzoxifene and its analogues were assayed in the standard ER competitive radioligand binding assay, using full length human recombinant ER-alpha and ...More data for this Ligand-Target Pair