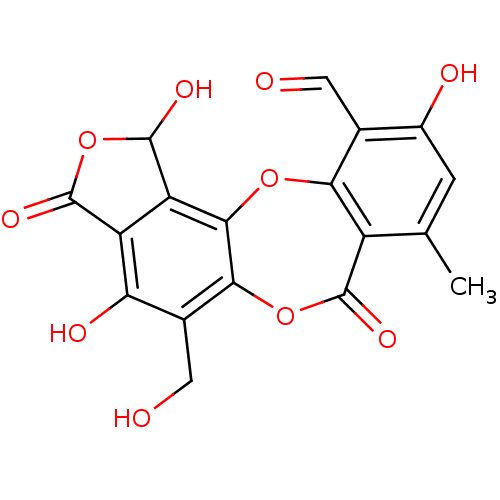
BDBM29673 1,4,10-Trihydroxy-5-hydroxymethyl-8-methyl-3,7-dioxo-1,3-dihydro-7H-benzo[e]furo[3'''',4'''':3,4]benzo[b][1,4]dioxepine-11-carbaldehyde::CHEMBL172439::NSC-87509::Salazinic acid, 2::cid_5320418
SMILES Cc1cc(O)c(C=O)c2Oc3c4C(O)OC(=O)c4c(O)c(CO)c3OC(=O)c12
InChI Key InChIKey=QQTKVXCQLZIJPP-UHFFFAOYSA-N
Data 9 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 29673
Found 9 hits for monomerid = 29673
Affinity DataIC50: 8.50E+4nMAssay Description:Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte...More data for this Ligand-Target Pair
Affinity DataIC50: 8.02E+3nMAssay Description:Project Title: A screen for modulators of human Rad51, a key DNA repair protein Application Number: MH084119 Assay Submitter: Dr. Alex Mazin Submitte...More data for this Ligand-Target Pair
TargetAmyloid-beta A4 precursor protein-binding family A member 1(Rattus norvegicus)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.25E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetVoltage-dependent N-type calcium channel subunit alpha-1B(Homo sapiens (Human))
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.48E+4nMAssay Description:Data Source: Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute Network...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+4nMAssay Description:Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 1More data for this Ligand-Target Pair
Affinity DataIC50: 1.60E+4nMAssay Description:Inhibitory concentration of compound against 3'-processing of HIV-1 integrase in experiment 2More data for this Ligand-Target Pair
Affinity DataIC50: 1.26E+4nMAssay Description:Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 1More data for this Ligand-Target Pair
Affinity DataIC50: 6.20E+3nMAssay Description:Inhibitory concentration of compound against strand transfer of HIV-1 integrase in experiment 2More data for this Ligand-Target Pair
TargetDual specificity mitogen-activated protein kinase kinase 6(Homo sapiens (Human))
Yale University School of Medicine
Yale University School of Medicine
Affinity DataIC50: 4.20E+4nMAssay Description:The labeled fragment can be distinguished from full-length MKK by a change in fluorescence polarization. MKK6 was used as a substrate because it cou...More data for this Ligand-Target Pair