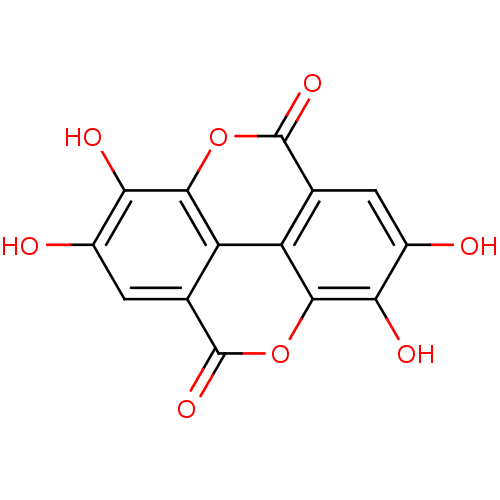
BDBM4078 6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0^{4,16}.0^{11,15}]hexadeca-1(14),4(16),5,7,11(15),12-hexaene-3,10-dione::CHEMBL6246::ELLAGIC ACID::Elagic Acid::Ellagic acid (18)::Ellagic acid dihydrate::MLS000069632::SMR000058244::cid_5281855
SMILES c1c2c-3c(c(c1O)O)OC(=O)c4c3c(c(c(c4)O)O)OC2=O
InChI Key InChIKey=AFSDNFLWKVMVRB-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 120 hits for monomerid = 4078
Found 120 hits for monomerid = 4078
Affinity DataIC50: 300nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration, which inhibits 50% of pp60c-src activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] ...More data for this Ligand-Target Pair
Affinity DataIC50: 600nMpH: 7.4 T: 2°CAssay Description:IC50 is the inhibitor concentration, which inhibits 50% of PKA activity that catalyzes the transfer of the terminal phosphate from [gamma-32P] label...More data for this Ligand-Target Pair
TargetHeat shock 70 kDa protein 1A(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 760nMpH: 7.0 T: 2°CAssay Description:Burnham Center for Chemical Genomics (BCCG) Burnham Institute for Medical Research (San Diego, CA) NIH Molecular Libraries Screening Centers Network ...More data for this Ligand-Target Pair
TargetHeat shock 70 kDa protein 1A(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.00E+5nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (San Diego, CA) NIH Molecular Libraries Screening Cen...More data for this Ligand-Target Pair
TargetAcetyl-CoA acetyltransferase/HMG-CoA reductase(Enterococcus faecalis)
Srmlsc
Curated by PubChem BioAssay
Srmlsc
Curated by PubChem BioAssay
Affinity DataIC50: 1.27E+6nMAssay Description:Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening C...More data for this Ligand-Target Pair
Affinity DataIC50: 142nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: F.M. Hoffmann, University ...More data for this Ligand-Target Pair
TargetEukaryotic translation initiation factor 4 gamma 1(Human)
Emory University
Curated by PubChem BioAssay
Emory University
Curated by PubChem BioAssay
Affinity DataIC50: 4.77E+4nMAssay Description:Dose Response Confirmation for Small Molecule Inhibitors of Eukaryotic Translation Initiation NIH Molecular Libraries Screening Centers Network [MLSC...More data for this Ligand-Target Pair
TargetInduced myeloid leukemia cell differentiation protein Mcl-1(Human)
Emory University
Curated by PubChem BioAssay
Emory University
Curated by PubChem BioAssay
Affinity DataIC50: 5.40E+4nMAssay Description:NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 6(Rat)
Sanford-Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Sanford-Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.95E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetHeat shock cognate 71 kDa protein(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 9.92E+4nMAssay Description:Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (San Diego, CA) NIH Molecular Libraries Screening Cen...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.23E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Target2'-phosphotransferase(Yeast)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 541nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetGuanyl-specific ribonuclease T1(Yellow koji mold)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 5.57E+4nMAssay Description:Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center (SRIMSC) Center Affiliation: The Scripps Research Institute (TS...More data for this Ligand-Target Pair
TargetM17 leucyl aminopeptidase(Plasmodium falciparum (isolate 3D7))
Srmlsc
Curated by PubChem BioAssay
Srmlsc
Curated by PubChem BioAssay
Affinity DataIC50: 1.38E+5nMAssay Description:Southern Research Molecular Libraries Screening Center (SRMLSC) Southern Research Institute (Birmingham, Alabama) NIH Molecular Libraries Screening...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 980nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.8Assay Description:The reaction mixture contained DNA (1.25 uM or as specified), NTP (110 uM or as specified), 50 mM NaCl, 150 mM potassium glutamate, buffer [20 mM CAP...More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:A range of natural products were screened for inhibition of PfGST by GST assay with CDNB as a substrate, using a 96-well SpectraMax 340 microplate sp...More data for this Ligand-Target Pair
Affinity DataIC50: 7.73E+3nMpH: 6.0Assay Description:PTP1B activity was measured by adding 2mM p-NPP and PTP1B in a 50 mM citrate buffer (pH 6.0, 0.1 M NaCl, 1 mMEDTA, and 1 mM dithiothreitol), with or ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMpH: 8.0 T: 2°CAssay Description:Briefly, 140 μL of sodium phosphate buffer (pH 8.0), 20 μL of each tested compound with different concentrations (4, 20, and 100 μM) a...More data for this Ligand-Target Pair
Affinity DataIC50: 2.20E+5nMAssay Description:Inhibition of cruzain in presence of 0.01% Triton X-100More data for this Ligand-Target Pair
Affinity DataIC50: 2.07E+4nMAssay Description:Inhibition of sEH (unknown origin) assessed as substrate PHOME hydrolysis after 1 hr by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.90E+3nMAssay Description:Inhibition of BACE1 (unknown origin)More data for this Ligand-Target Pair
TargetcAMP-dependent protein kinase catalytic subunit alpha/beta/gamma(Human)
Università
Curated by ChEMBL
Università
Curated by ChEMBL
Affinity DataIC50: 3.50E+3nMAssay Description:Inhibition of PKAMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of NPM-ALKMore data for this Ligand-Target Pair
TargetCyclin-A1/Cyclin-A2/Cyclin-dependent kinase 2(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 3.39E+3nMAssay Description:Inhibition of human recombinant CDK2/CyclinA expressed in Sf9 cells using histone H1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+3nMAssay Description:Inhibition of human recombinant aurora-B expressed in Sf9 cells using tetra(LRRWSLG) as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetCyclin-dependent kinase 4/G1/S-specific cyclin-D1(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 1.09E+3nMAssay Description:Inhibition of human recombinant CDK4/CyclinD1 expressed in Sf9 cells using RB-CTF as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 340nMAssay Description:Inhibition of human recombinant INS-R expressed in Sf9 cells using poly(A,E,K,Y)6:2:5:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of rat spleen LynMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of RETMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of FLT3More data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Inhibition of rat spleen SYKMore data for this Ligand-Target Pair
Affinity DataIC50: 9.40E+3nMAssay Description:Inhibition of rat spleen FGRMore data for this Ligand-Target Pair
TargetDual specificity tyrosine-phosphorylation-regulated kinase 1A(Human)
Università
Curated by ChEMBL
Università
Curated by ChEMBL
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of human recombinant DYRK1aMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of rat spleen CSKMore data for this Ligand-Target Pair
Affinity DataIC50: 7.50E+3nMAssay Description:Inhibition of human recombinant GSK3-betaMore data for this Ligand-Target Pair
Affinity DataIC50: 40nMAssay Description:Inhibition of GST-fused human recombinant CK2alpha expressed in Escherichia coli HMS174 (DE3)More data for this Ligand-Target Pair
Affinity DataIC50: 7.71E+4nMAssay Description:Inhibition of human glutathione S-transferase PMore data for this Ligand-Target Pair
TargetGlutathione S-transferase(malaria parasite P. falciparum)
Justus-Liebig-University
Curated by ChEMBL
Justus-Liebig-University
Curated by ChEMBL
Affinity DataIC50: 7.44E+4nMAssay Description:Inhibition of Plasmodium falciparum glutathione S-transferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.28E+4nMAssay Description:Inhibition of human glutathione reductase by spectrophotometerMore data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human recombinant aldose reductase using D-glyceraldehyde as substrate preincubated for 10 mins before substrate addition measured for ...More data for this Ligand-Target Pair
TargetRAC-alpha serine/threonine-protein kinase(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 3.34E+3nMAssay Description:Inhibition of human recombinant AKT1 expressed in Sf9 cells using GSK3(14-27) as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetNUAK family SNF1-like kinase 1(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 510nMAssay Description:Inhibition of human recombinant ARK5 expressed in Sf9 cells assessed as autophosphorylation after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 2.48E+3nMAssay Description:Inhibition of human recombinant aurora-A expressed in Sf9 cells using tetra(LRRWSLG) as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetSerine/threonine-protein kinase B-raf(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 1.76E+3nMAssay Description:Inhibition of human recombinant B-RAF expressed in Sf9 cells using MEK1 KM as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetEpidermal growth factor receptor(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 690nMAssay Description:Inhibition of human recombinant EGF-R expressed in Sf9 cells using poly(E,Y)4:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 1.83E+3nMAssay Description:Inhibition of human recombinant EPHB4 expressed in Sf9 cells using poly(E,Y)4:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetReceptor tyrosine-protein kinase erbB-2(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 3.54E+3nMAssay Description:Inhibition of human recombinant ERBB2 expressed in Sf9 cells using poly(E,Y)4:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.11E+3nMAssay Description:Inhibition of human recombinant FAK expressed in Sf9 cells using poly(E,Y)4:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
TargetInsulin-like growth factor 1 receptor(Human)
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Westf£Lische Wilhelms-Universit£T M£Nster
Curated by ChEMBL
Affinity DataIC50: 250nMAssay Description:Inhibition of human recombinant IGF1R expressed in Sf9 cells using poly(E,Y)4:1 as substrate after 80 mins by scintillation countingMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)