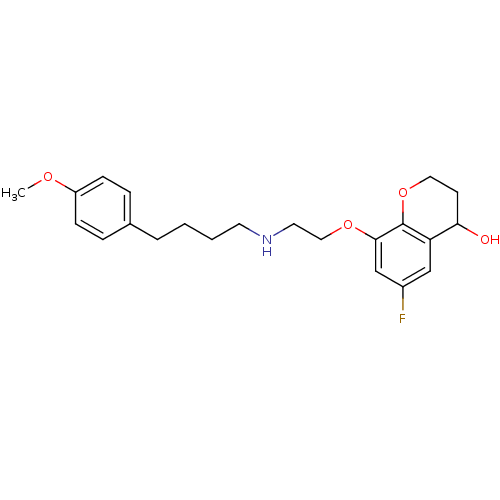
BDBM50065563 6-Fluoro-8-{2-[4-(4-methoxy-phenyl)-butylamino]-ethoxy}-chroman-4-ol::CHEMBL96714
SMILES COc1ccc(CCCCNCCOc2cc(F)cc3C(O)CCOc23)cc1
InChI Key InChIKey=GQVNYCJRZSTERX-UHFFFAOYSA-N
Data 4 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50065563
Found 4 hits for monomerid = 50065563
Affinity DataKi: 0.724nMAssay Description:Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand.More data for this Ligand-Target Pair
Affinity DataKi: 0.724nMAssay Description:Binding affinity against dopamine receptor D2 on rat striatum using [3H]-spiperone as radioligand.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
Yamanouchi Pharmaceutical
Curated by ChEMBL
Yamanouchi Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand.More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1A(Rattus norvegicus (rat))
Yamanouchi Pharmaceutical
Curated by ChEMBL
Yamanouchi Pharmaceutical
Curated by ChEMBL
Affinity DataKi: 2.10nMAssay Description:Binding affinity against 5-hydroxytryptamine 1A receptor on rat hippocampus using [3H]-8-OH-DPAT as radioligand.More data for this Ligand-Target Pair