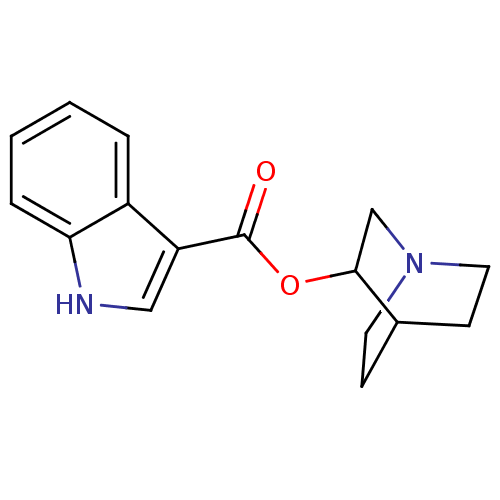
BDBM50096713 1H-Indole-3-carboxylic acid 1-aza-bicyclo[2.2.2]oct-3-yl ester::CHEMBL108799
SMILES O=C(OC1CN2CCC1CC2)c1c[nH]c2ccccc12
InChI Key InChIKey=VUADLQGWANQBFT-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50096713
Found 4 hits for monomerid = 50096713
Affinity DataKi: 2.30nMAssay Description:In vitro Binding affinity towards alpha-7 nAChR was determinedMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-4/beta-2(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataKi: 2.60E+3nMAssay Description:In vitro Binding affinity towards alpha-1-beta-1-gamma-delta nAChR was determinedMore data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Homo sapiens (Human))
Astrazeneca
Curated by ChEMBL
Astrazeneca
Curated by ChEMBL
Affinity DataEC50: 580nMAssay Description:Effective concentration that causes inhibition of alpha-7 nAChR, was determined. Values are expressed as EC50 +/- SEM.More data for this Ligand-Target Pair
TargetNeuronal acetylcholine receptor subunit alpha-7(Rattus norvegicus (Rat))
Universit£
Curated by ChEMBL
Universit£
Curated by ChEMBL
Affinity DataEC50: 580nMAssay Description:Partial agonist activity at rat alpha-7 nicotinic acetylcholine receptor expressed in Xenopus oocytes assessed as induction of currentsMore data for this Ligand-Target Pair