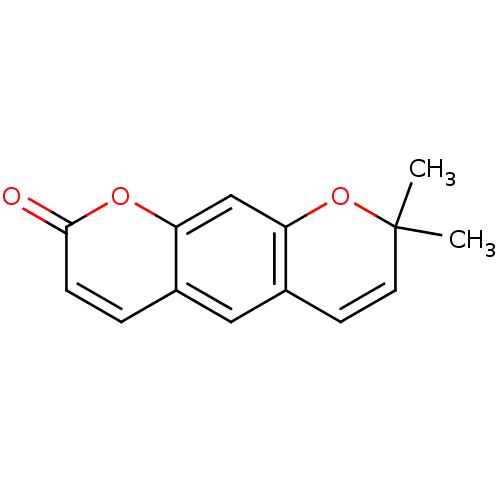
BDBM50292575 8,8-Dimethyl-8H-pyrano[3,2-g]chromen-2-one::CHEMBL303846::XANTHYLETIN::cid_65188
SMILES CC1(C)Oc2cc3oc(=O)ccc3cc2C=C1
InChI Key InChIKey=QOTBQNVNUBKJMS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 50292575
Found 10 hits for monomerid = 50292575
TargetFructose-bisphosphate aldolase(Mycobacterium tuberculosis (strain ATCC 25618 / H3...)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataKi: 7.51E+3nMAssay Description:Inhibition of human carbonic anhydrase 9 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 8.36E+3nMAssay Description:Inhibition of human carbonic anhydrase 13 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 9.18E+3nMAssay Description:Inhibition of human carbonic anhydrase 7 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.15E+4nMAssay Description:Inhibition of human carbonic anhydrase 1 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.57E+4nMAssay Description:Inhibition of human carbonic anhydrase 12 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+5nMAssay Description:Inhibition of human carbonic anhydrase 2 preincubated for 6 hrs by CO2 hydration stopped-flow assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+5nMAssay Description:Inhibition of AChE by spectrophotometryMore data for this Ligand-Target Pair
TargetGlyceraldehyde-3-phosphate dehydrogenase(Human)
Universidade Federal De Minas Gerais
Curated by ChEMBL
Universidade Federal De Minas Gerais
Curated by ChEMBL
Affinity DataIC50: 1.95E+5nMAssay Description:Inhibitory concentration against glyceraldehyde-3-phosphate dehydrogenase was determined as log 1/IC50More data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of recombinant human BACE1 using Rh-EVNLDAEFK as substrate after 60 mins by fluorescence quenching assayMore data for this Ligand-Target Pair