BDBM50382163 CHEMBL2023820
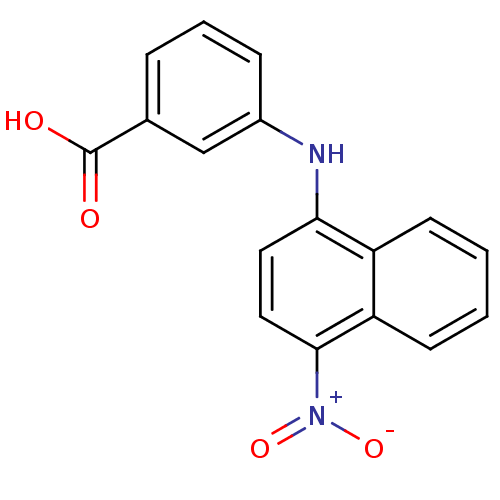
SMILES c1ccc2c(c1)c(ccc2[N+](=O)[O-])Nc3cccc(c3)C(=O)O
InChI Key InChIKey=ZLIDMWNTWSBTSM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50382163
Found 14 hits for monomerid = 50382163
TargetAldo-keto reductase family 1 member C3(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 80nMAssay Description:Inhibition of recombinant AKR1C3 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C3(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 86nMAssay Description:Inhibition of human AKR1C3 using S-tetralol as substrate in presence of NADP by fluorescence methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Antagonistic activity at AR in human 22Rv1 cells assessed as reduction in cell number by CCK8 assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+3nMAssay Description:Antagonist activity at androgen receptor expressed in HeLa-AR3A-PSA-(ARE)4-Luc13 cells assessed as rightward shift of DHT-induced response incubated ...More data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+3nMAssay Description:Displacement of [3H]-R1881 from androgen receptor (unknown origin) expressed in human HeLa cells harboring AR3A-PSA-(ARE)4-Luc13 incubated for 2 hrs ...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 8.17E+3nMAssay Description:Inhibition of recombinant AKR1C2 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 8.17E+3nMAssay Description:Inhibition of recombinant AKR1C4 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.11E+4nMAssay Description:Inhibition of recombinant AKR1C1 assessed as enzyme catalyzed oxidation of S-tetralol by fluorimetric assayMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Antagonist activity at androgen receptor (unknown origin) expressed in human HeLa cells harboring AR3A-PSA-(ARE)4-Luc13 in presence of DHT by BrightG...More data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C2(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 2.47E+4nMAssay Description:Inhibition of human AKR1C2 using S-tetralol as substrate in presence of NADP by fluorescence methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C4(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 3.06E+4nMAssay Description:Inhibition of human AKR1C4 using S-tetralol as substrate in presence of NADP by fluorescence methodMore data for this Ligand-Target Pair
TargetAldo-keto reductase family 1 member C1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 4.13E+4nMAssay Description:Inhibition of human AKR1C1 using S-tetralol as substrate in presence of NADP by fluorescence methodMore data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 1(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant COX1More data for this Ligand-Target Pair
TargetProstaglandin G/H synthase 2(Human)
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Perelman School of Medicine University of Pennsylvania
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of recombinant COX2More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)