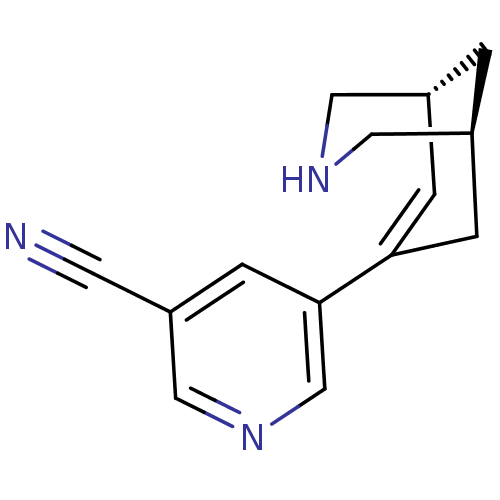
BDBM50398847 CHEMBL2177535
SMILES N#Cc1cncc(c1)C1=C[C@@H]2CNC[C@@H](C2)C1
InChI Key InChIKey=DEXLAAAKAUMETM-MNOVXSKESA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50398847
Found 6 hits for monomerid = 50398847
Affinity DataKi: 0.300nMAssay Description:Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 0.600nMAssay Description:Displacement of [3H]nicotine from human alpha4beta2 nAChR expressed in human SH-EP1 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 79nMAssay Description:Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cellsMore data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Displacement of [3H]epibatidine from human alpha7 nAChR expressed in human HEK/RIC3 cellsMore data for this Ligand-Target Pair
Affinity DataEC50: 107nMAssay Description:Agonist activity at human alpha4beta2 nAChR high sensitivity form expressed in human SH-EP1 cells assessed as increase in calcium flux by FLIPRMore data for this Ligand-Target Pair
Affinity DataEC50: 343nMAssay Description:Agonist activity at human alpha4beta2 nAChR low sensitivity form expressed in human SH-EP1 cells assessed as increase in calcium flux by FLIPRMore data for this Ligand-Target Pair