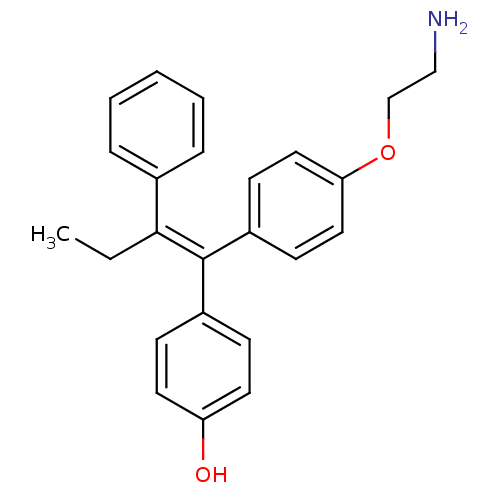
BDBM50435004 CHEMBL2386284
SMILES CC\C(=C(/c1ccc(O)cc1)c1ccc(OCCN)cc1)c1ccccc1
InChI Key InChIKey=YCQBLTPGQSYLHD-VHXPQNKSSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 50435004
Found 9 hits for monomerid = 50435004
Affinity DataKi: 442nMAssay Description:Inhibition of human recombinant microsomal aromatase using 7-methoxy-4-trifluoromethylcoumarin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataKi: 442nMAssay Description:Inhibition of human recombinant microsomal aromatase using 7-methoxy-4-trifluoromethylcoumarin as substrate by Lineweaver-Burk plot analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 17nMAssay Description:Displacement of fluorescent ES2 from recombinant human ERalpha after 2 hrs in absence of light by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 28nMAssay Description:Displacement of fluorescent ES2 from recombinant human ERbeta after 2 hrs in absence of light by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 90nMAssay Description:Inhibition of human recombinant placental aromataseMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+3nMAssay Description:Inhibition of human recombinant microsomal aromatase-mediated 7-methoxy-4-trifluoromethylcoumarin conversion to 7-hydroxytrifluoromethylcoumarin prei...More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+3nMAssay Description:Inhibition of human recombinant microsomal aromatase-mediated 7-methoxy-4-trifluoromethylcoumarin conversion to 7-hydroxytrifluoromethylcoumarin prei...More data for this Ligand-Target Pair
Affinity DataIC50: 1.03E+3nMAssay Description:Inhibition of recombinant human microsomal CYP19 using MFC as substrate measured after 30 mins by fluorometric analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human placental microsomal aromatase using testosterone as substrate assessed as formation of estradiol after 10 minsMore data for this Ligand-Target Pair