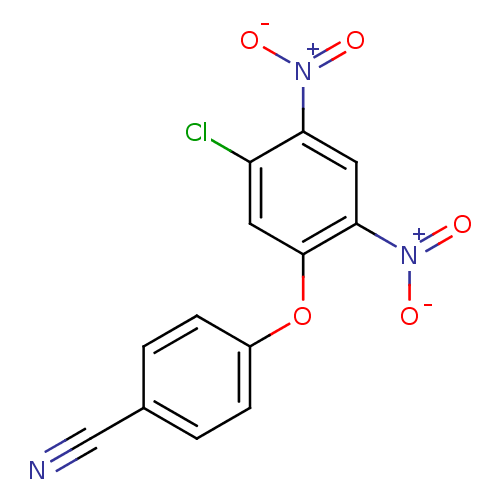
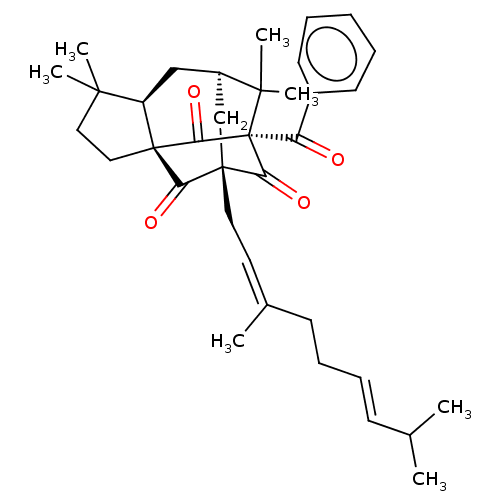
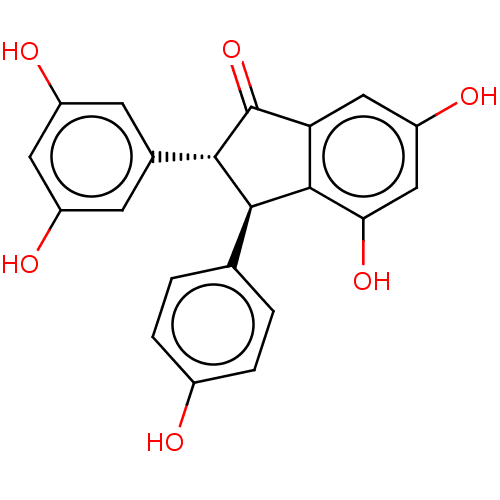
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.20E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: 2.40E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase Mer(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of MerMore data for this Ligand-Target Pair
Affinity DataIC50: 9.80E+3nMAssay Description:Inhibition of human erythrocytes acetylcholinesterase after 30 mins using S-acetylthiocholine iodide substrate by spectrophotometry based Ellman meth...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
Affinity DataIC50: 2.54E+4nMAssay Description:Inhibition of human erythrocytes acetylcholinesterase after 30 mins using S-acetylthiocholine iodide substrate by spectrophotometry based Ellman meth...More data for this Ligand-Target Pair
Affinity DataIC50: 2.64E+4nMAssay Description:Inhibition of human erythrocytes acetylcholinesterase after 30 mins using S-acetylthiocholine iodide substrate by spectrophotometry based Ellman meth...More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor TYRO3(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of Tyro3More data for this Ligand-Target Pair
TargetTyrosine-protein kinase receptor UFO(Homo sapiens (Human))
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Beijing Institute Of Pharmacology & Toxicology
Curated by ChEMBL
Affinity DataIC50: >3.00E+4nMAssay Description:Inhibition of AxlMore data for this Ligand-Target Pair
Affinity DataIC50: 3.72E+4nMAssay Description:Inhibition of human erythrocytes acetylcholinesterase after 30 mins using S-acetylthiocholine iodide substrate by spectrophotometry based Ellman meth...More data for this Ligand-Target Pair
Affinity DataEC50: 4.58E+4nMAssay Description:Agonist activity at ERbeta (248 to 510 amino acid residues) (unknown origin) assessed as induction of interaction with SRC1 after 24 hrs by yeast two...More data for this Ligand-Target Pair
Affinity DataEC50: 4.70E+4nMAssay Description:Agonist activity at ERbeta (248 to 510 amino acid residues) (unknown origin) assessed as induction of interaction with SRC1 after 24 hrs by yeast two...More data for this Ligand-Target Pair
Affinity DataEC50: 5.65E+3nMAssay Description:Agonist activity at ERbeta (248 to 510 amino acid residues) (unknown origin) assessed as induction of interaction with SRC1 after 24 hrs by yeast two...More data for this Ligand-Target Pair