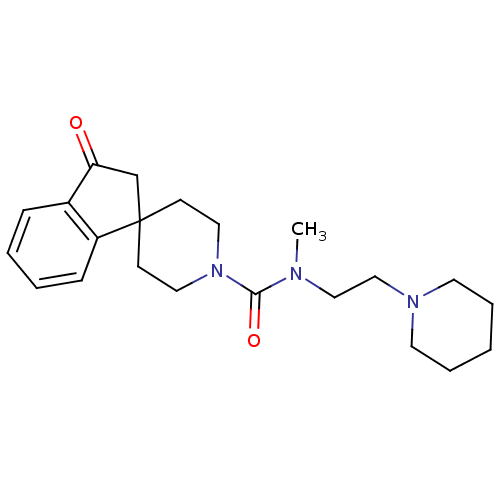
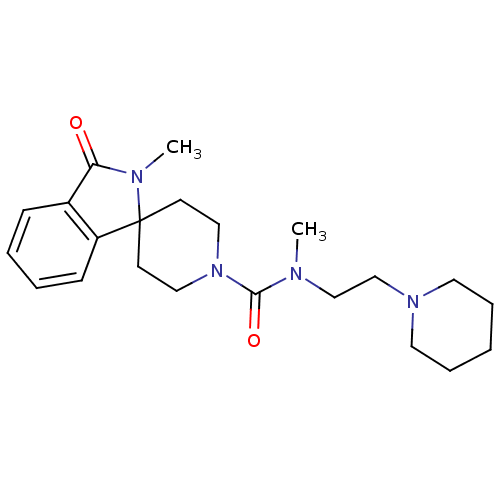
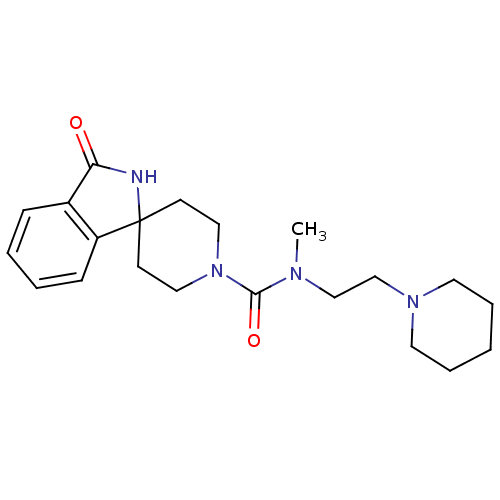
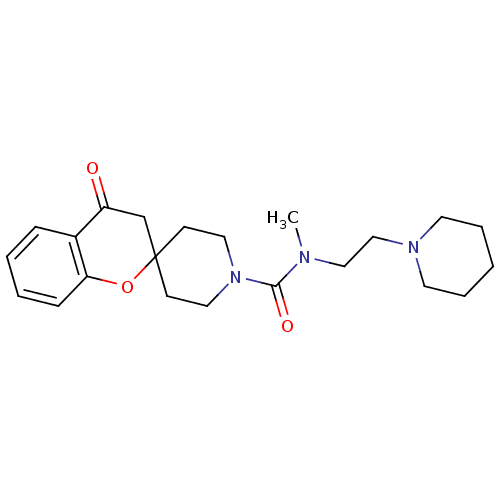
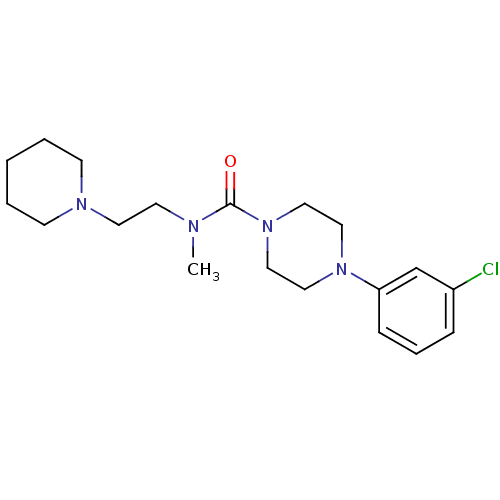
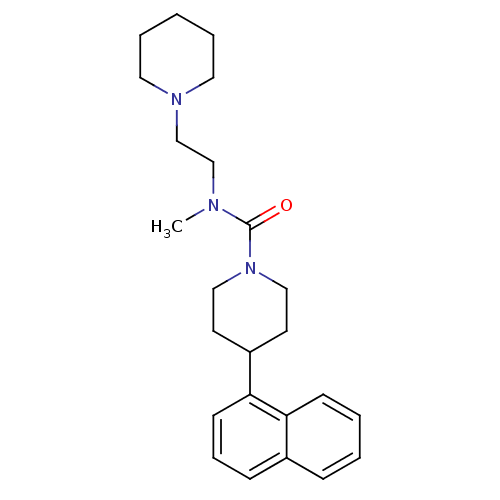
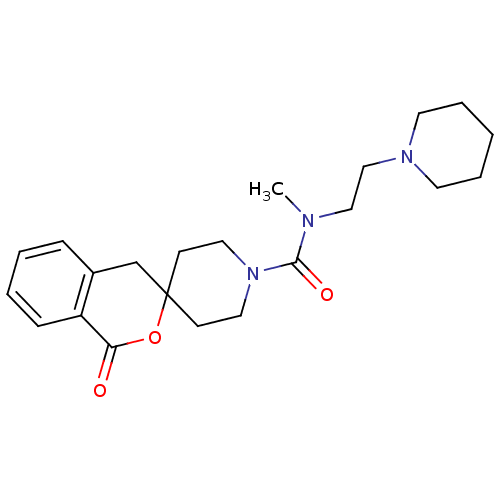
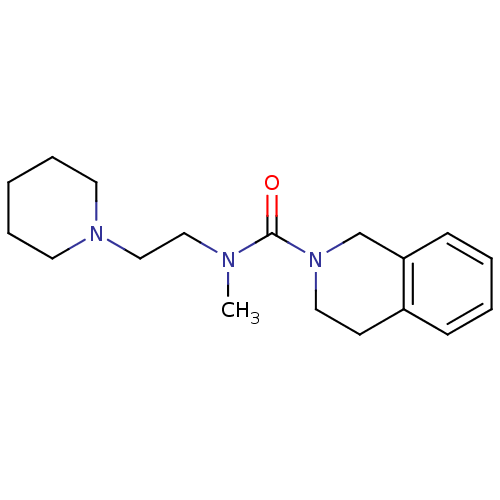
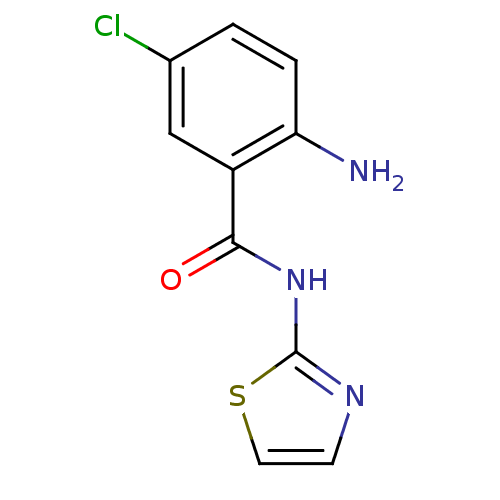
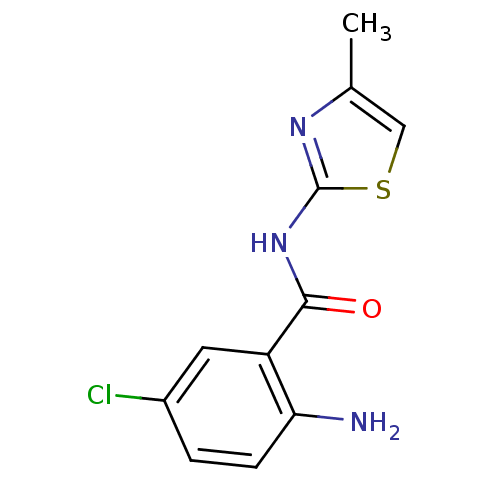
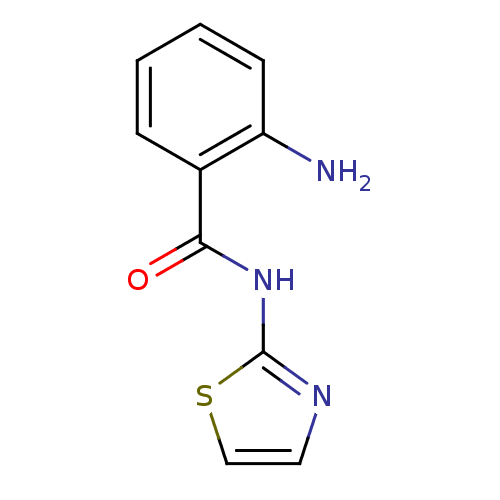
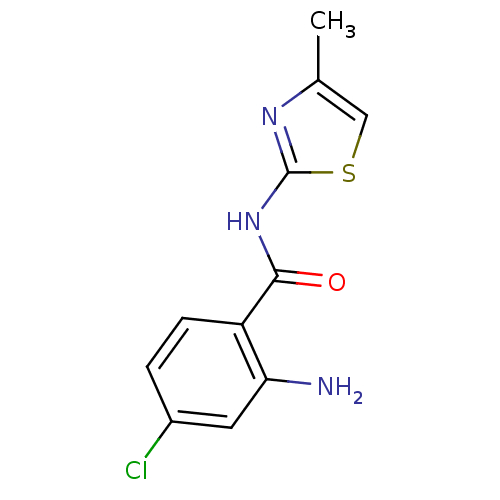
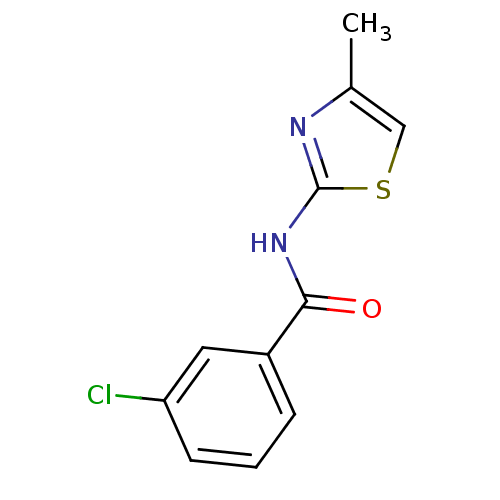
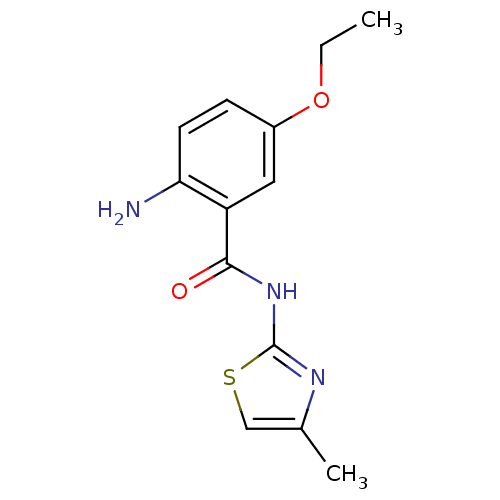
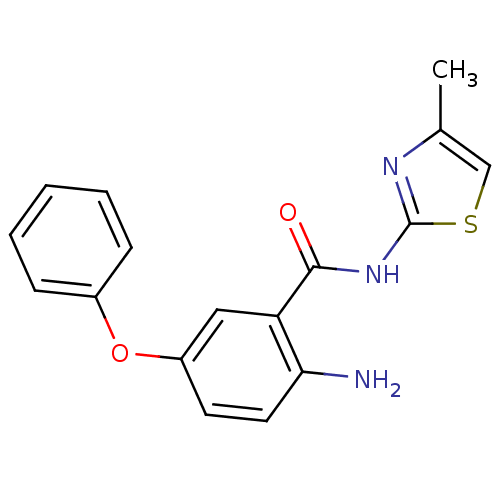
Affinity DataIC50: 0.540nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 0.720nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 45nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 68nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 98nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 160nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 237nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 267nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 297nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 343nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 380nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 387nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 393nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 506nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 530nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 560nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 680nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 783nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: 893nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+3nMAssay Description:Inverse agonist activity at human cloned histamine H3 receptor assessed as inhibition of (R)-alpha-methylhistamine-induced [35S]GTPgammaS bindingMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H4 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H2 receptor (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H1 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth...More data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+4nMAssay Description:Inhibition of histamine H4 receptor (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Homo sapiens (Human))
Tsukuba Research Institute
Curated by ChEMBL
Tsukuba Research Institute
Curated by ChEMBL
Affinity DataIC50: >1.00E+4nMAssay Description:Displacement of [35S]N-[(4R)-1'-[(2R)-6-cyano-1,2,3,4-tetrahydro-2-naphthalenyl]-3,4-dihydro-4-hydroxyspiro[2H-1-benzopyran-2,4'-piperidin]-6-yl]meth...More data for this Ligand-Target Pair
Affinity DataEC50: 1.10E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 2.60E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 6.50E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 1.40E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: >3.00E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+4nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 6.30E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 6.80E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 1.70E+3nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 10 mM glucoseMore data for this Ligand-Target Pair
Affinity DataEC50: 700nMAssay Description:Activation of human glucokinase by glucose-6-phosphate dehydrogenase coupled continuous spectrophotometric assay in presence of 2.5 mM glucoseMore data for this Ligand-Target Pair